RNA-Binding Proteins with Mixed Charge Domains Self-Assemble and Aggregate in Alzheimer's Disease*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Splicing Regulatory Factors in Breast Cancer Hallmarks and Disease Progression
www.oncotarget.com Oncotarget, 2019, Vol. 10, (No. 57), pp: 6021-6037 Review Splicing regulatory factors in breast cancer hallmarks and disease progression Esmee Koedoot1, Liesanne Wolters1, Bob van de Water1 and Sylvia E. Le Dévédec1 1Division of Drug Discovery and Safety, LACDR, Leiden University, Leiden, The Netherlands Correspondence to: Sylvia E. Le Dévédec, email: [email protected] Keywords: hallmarks of cancer; breast cancer; alternative splicing; splice factors; RNA sequencing Received: April 23, 2019 Accepted: August 29, 2019 Published: October 15, 2019 Copyright: Koedoot et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT By regulating transcript isoform expression levels, alternative splicing provides an additional layer of protein control. Recent studies show evidence that cancer cells use different splicing events to fulfill their requirements in order to develop, progress and metastasize. However, there has been less attention for the role of the complex catalyzing the complicated multistep splicing reaction: the spliceosome. The spliceosome consists of multiple sub-complexes in total comprising 244 proteins or splice factors and 5 associated RNA molecules. Here we discuss the role of splice factors in the oncogenic processes tumors cells need to fulfill their oncogenic properties (the so-called the hallmarks of cancer). Despite the fact that splice factors have been investigated only recently, they seem to play a prominent role in already five hallmarks of cancer: angiogenesis, resisting cell death, sustaining proliferation, deregulating cellular energetics and invasion and metastasis formation by affecting major signaling pathways such as epithelial-to-mesenchymal transition, the Warburg effect, DNA damage response and hormone receptor dependent proliferation. -

Miasdb: a Database of Molecular Interactions Associated with Alternative Splicing of Human Pre-Mrnas
RESEARCH ARTICLE MiasDB: A Database of Molecular Interactions Associated with Alternative Splicing of Human Pre-mRNAs Yongqiang Xing1, Xiujuan Zhao1, Tao Yu2, Dong Liang1, Jun Li1, Guanyun Wei1, Guoqing Liu1, Xiangjun Cui1, Hongyu Zhao1, Lu Cai1* 1 School of Life Science and Technology, Inner Mongolia University of Science and Technology, Baotou, 014010, China, 2 School of Science, Inner Mongolia University of Science and Technology, Baotou, 014010, China a11111 * [email protected] Abstract Alternative splicing (AS) is pervasive in human multi-exon genes and is a major contributor to expansion of the transcriptome and proteome diversity. The accurate recognition of alter- OPEN ACCESS native splice sites is regulated by information contained in networks of protein-protein and Citation: Xing Y, Zhao X, Yu T, Liang D, Li J, Wei G, protein-RNA interactions. However, the mechanisms leading to splice site selection are not et al. (2016) MiasDB: A Database of Molecular fully understood. Although numerous databases have been built to describe AS, molecular Interactions Associated with Alternative Splicing of Human Pre-mRNAs. PLoS ONE 11(5): e0155443. interaction databases associated with AS have only recently emerged. In this study, we doi:10.1371/journal.pone.0155443 present a new database, MiasDB, that provides a description of molecular interactions Editor: Ruben Artero, University of Valencia, SPAIN associated with human AS events. This database covers 938 interactions between human splicing factors, RNA elements, transcription factors, kinases and modified histones for 173 Received: November 19, 2015 human AS events. Every entry includes the interaction partners, interaction type, experi- Accepted: April 28, 2016 mental methods, AS type, tissue specificity or disease-relevant information, a simple Published: May 11, 2016 description of the functionally tested interaction in the AS event and references. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

1 AGING Supplementary Table 2
SUPPLEMENTARY TABLES Supplementary Table 1. Details of the eight domain chains of KIAA0101. Serial IDENTITY MAX IN COMP- INTERFACE ID POSITION RESOLUTION EXPERIMENT TYPE number START STOP SCORE IDENTITY LEX WITH CAVITY A 4D2G_D 52 - 69 52 69 100 100 2.65 Å PCNA X-RAY DIFFRACTION √ B 4D2G_E 52 - 69 52 69 100 100 2.65 Å PCNA X-RAY DIFFRACTION √ C 6EHT_D 52 - 71 52 71 100 100 3.2Å PCNA X-RAY DIFFRACTION √ D 6EHT_E 52 - 71 52 71 100 100 3.2Å PCNA X-RAY DIFFRACTION √ E 6GWS_D 41-72 41 72 100 100 3.2Å PCNA X-RAY DIFFRACTION √ F 6GWS_E 41-72 41 72 100 100 2.9Å PCNA X-RAY DIFFRACTION √ G 6GWS_F 41-72 41 72 100 100 2.9Å PCNA X-RAY DIFFRACTION √ H 6IIW_B 2-11 2 11 100 100 1.699Å UHRF1 X-RAY DIFFRACTION √ www.aging-us.com 1 AGING Supplementary Table 2. Significantly enriched gene ontology (GO) annotations (cellular components) of KIAA0101 in lung adenocarcinoma (LinkedOmics). Leading Description FDR Leading Edge Gene EdgeNum RAD51, SPC25, CCNB1, BIRC5, NCAPG, ZWINT, MAD2L1, SKA3, NUF2, BUB1B, CENPA, SKA1, AURKB, NEK2, CENPW, HJURP, NDC80, CDCA5, NCAPH, BUB1, ZWILCH, CENPK, KIF2C, AURKA, CENPN, TOP2A, CENPM, PLK1, ERCC6L, CDT1, CHEK1, SPAG5, CENPH, condensed 66 0 SPC24, NUP37, BLM, CENPE, BUB3, CDK2, FANCD2, CENPO, CENPF, BRCA1, DSN1, chromosome MKI67, NCAPG2, H2AFX, HMGB2, SUV39H1, CBX3, TUBG1, KNTC1, PPP1CC, SMC2, BANF1, NCAPD2, SKA2, NUP107, BRCA2, NUP85, ITGB3BP, SYCE2, TOPBP1, DMC1, SMC4, INCENP. RAD51, OIP5, CDK1, SPC25, CCNB1, BIRC5, NCAPG, ZWINT, MAD2L1, SKA3, NUF2, BUB1B, CENPA, SKA1, AURKB, NEK2, ESCO2, CENPW, HJURP, TTK, NDC80, CDCA5, BUB1, ZWILCH, CENPK, KIF2C, AURKA, DSCC1, CENPN, CDCA8, CENPM, PLK1, MCM6, ERCC6L, CDT1, HELLS, CHEK1, SPAG5, CENPH, PCNA, SPC24, CENPI, NUP37, FEN1, chromosomal 94 0 CENPL, BLM, KIF18A, CENPE, MCM4, BUB3, SUV39H2, MCM2, CDK2, PIF1, DNA2, region CENPO, CENPF, CHEK2, DSN1, H2AFX, MCM7, SUV39H1, MTBP, CBX3, RECQL4, KNTC1, PPP1CC, CENPP, CENPQ, PTGES3, NCAPD2, DYNLL1, SKA2, HAT1, NUP107, MCM5, MCM3, MSH2, BRCA2, NUP85, SSB, ITGB3BP, DMC1, INCENP, THOC3, XPO1, APEX1, XRCC5, KIF22, DCLRE1A, SEH1L, XRCC3, NSMCE2, RAD21. -

PRPF40A As a Potential Diagnostic and Prognostic Marker Is Upregulated in Pancreatic Cancer Tissues and Cell Lines: an Integrated Bioinformatics Data Analysis
OncoTargets and Therapy Dovepress open access to scientific and medical research Open Access Full Text Article ORIGINAL RESEARCH PRPF40A as a potential diagnostic and prognostic marker is upregulated in pancreatic cancer tissues and cell lines: an integrated bioinformatics data analysis This article was published in the following Dove Press journal: OncoTargets and Therapy Zhen Huo* Background: Pre-mRNA processing factor 40 homolog A (PRPF40A) is an important Shuyu Zhai* protein involved in pre-mRNA splicing and is expressed in a variety of cell types. Yuanchi Weng* However, the function of PRPF40A in pancreatic cancer remains unclear. Therefore, our Hao Qian study is to investigate the role of PRPF40A in the pathogenesis of pancreatic cancer. Xiaomei Tang Materials and methods: We extracted expression data and clinical information of Yusheng Shi PRPF40A from different online databases, including the Cancer Genome Atlas (TCGA), Oncomine and the Gene Expression Omnibus (GEO). Subsequently, samples were collected Xiaxing Deng from patients to validate gene expression using qPCR, Western blotting and immunohisto- Yue Wang chemical (IHC) analyses. Receiver operating characteristic (ROC) and Kaplan-Meier curve Baiyong Shen were used to evaluate the diagnostic and prognostic potential. Colony formation assays and Department of General Surgery, Ruijin CCK-8 assays were performed to measure the proliferative capacity of pancreatic cancer. Hospital, Shanghai Jiao Tong University Finally, gene ontology (GO) and pathway enrichment analyses of co-expressed genes of School of Medicine, Shanghai 200025, People’s Republic of China PRPF40A were conducted using the Database for Annotation, Visualization and Integrated Discovery (DAVID). *These authors contributed equally to Results: We found that PRPF40A was upregulated based on data from both the online this work databases and our samples. -

WO 2019/079361 Al 25 April 2019 (25.04.2019) W 1P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2019/079361 Al 25 April 2019 (25.04.2019) W 1P O PCT (51) International Patent Classification: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, C12Q 1/68 (2018.01) A61P 31/18 (2006.01) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, C12Q 1/70 (2006.01) HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, (21) International Application Number: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, PCT/US2018/056167 OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (22) International Filing Date: SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 16 October 2018 (16. 10.2018) TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (26) Publication Language: English GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, (30) Priority Data: UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, 62/573,025 16 October 2017 (16. 10.2017) US TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, ΓΕ , IS, IT, LT, LU, LV, (71) Applicant: MASSACHUSETTS INSTITUTE OF MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, TECHNOLOGY [US/US]; 77 Massachusetts Avenue, TR), OAPI (BF, BJ, CF, CG, CI, CM, GA, GN, GQ, GW, Cambridge, Massachusetts 02139 (US). -

Supplementary Materials
Supplementary Materials COMPARATIVE ANALYSIS OF THE TRANSCRIPTOME, PROTEOME AND miRNA PROFILE OF KUPFFER CELLS AND MONOCYTES Andrey Elchaninov1,3*, Anastasiya Lokhonina1,3, Maria Nikitina2, Polina Vishnyakova1,3, Andrey Makarov1, Irina Arutyunyan1, Anastasiya Poltavets1, Evgeniya Kananykhina2, Sergey Kovalchuk4, Evgeny Karpulevich5,6, Galina Bolshakova2, Gennady Sukhikh1, Timur Fatkhudinov2,3 1 Laboratory of Regenerative Medicine, National Medical Research Center for Obstetrics, Gynecology and Perinatology Named after Academician V.I. Kulakov of Ministry of Healthcare of Russian Federation, Moscow, Russia 2 Laboratory of Growth and Development, Scientific Research Institute of Human Morphology, Moscow, Russia 3 Histology Department, Medical Institute, Peoples' Friendship University of Russia, Moscow, Russia 4 Laboratory of Bioinformatic methods for Combinatorial Chemistry and Biology, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences, Moscow, Russia 5 Information Systems Department, Ivannikov Institute for System Programming of the Russian Academy of Sciences, Moscow, Russia 6 Genome Engineering Laboratory, Moscow Institute of Physics and Technology, Dolgoprudny, Moscow Region, Russia Figure S1. Flow cytometry analysis of unsorted blood sample. Representative forward, side scattering and histogram are shown. The proportions of negative cells were determined in relation to the isotype controls. The percentages of positive cells are indicated. The blue curve corresponds to the isotype control. Figure S2. Flow cytometry analysis of unsorted liver stromal cells. Representative forward, side scattering and histogram are shown. The proportions of negative cells were determined in relation to the isotype controls. The percentages of positive cells are indicated. The blue curve corresponds to the isotype control. Figure S3. MiRNAs expression analysis in monocytes and Kupffer cells. Full-length of heatmaps are presented. -

Nuclear PTEN Safeguards Pre-Mrna Splicing to Link Golgi Apparatus for Its Tumor Suppressive Role
ARTICLE DOI: 10.1038/s41467-018-04760-1 OPEN Nuclear PTEN safeguards pre-mRNA splicing to link Golgi apparatus for its tumor suppressive role Shao-Ming Shen1, Yan Ji2, Cheng Zhang1, Shuang-Shu Dong2, Shuo Yang1, Zhong Xiong1, Meng-Kai Ge1, Yun Yu1, Li Xia1, Meng Guo1, Jin-Ke Cheng3, Jun-Ling Liu1,3, Jian-Xiu Yu1,3 & Guo-Qiang Chen1 Dysregulation of pre-mRNA alternative splicing (AS) is closely associated with cancers. However, the relationships between the AS and classic oncogenes/tumor suppressors are 1234567890():,; largely unknown. Here we show that the deletion of tumor suppressor PTEN alters pre-mRNA splicing in a phosphatase-independent manner, and identify 262 PTEN-regulated AS events in 293T cells by RNA sequencing, which are associated with significant worse outcome of cancer patients. Based on these findings, we report that nuclear PTEN interacts with the splicing machinery, spliceosome, to regulate its assembly and pre-mRNA splicing. We also identify a new exon 2b in GOLGA2 transcript and the exon exclusion contributes to PTEN knockdown-induced tumorigenesis by promoting dramatic Golgi extension and secretion, and PTEN depletion significantly sensitizes cancer cells to secretion inhibitors brefeldin A and golgicide A. Our results suggest that Golgi secretion inhibitors alone or in combination with PI3K/Akt kinase inhibitors may be therapeutically useful for PTEN-deficient cancers. 1 Department of Pathophysiology, Key Laboratory of Cell Differentiation and Apoptosis of Chinese Ministry of Education, Shanghai Jiao Tong University School of Medicine (SJTU-SM), Shanghai 200025, China. 2 Institute of Health Sciences, Shanghai Institutes for Biological Sciences of Chinese Academy of Sciences and SJTU-SM, Shanghai 200025, China. -
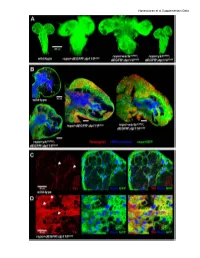
Supplementary Data Vigneswaran Et Al Supplementary Data
Vigneswaran et al Supplementary Data Vigneswaran et al Supplementary Data Figure S1: Yki is required for EGFR-PI3K-driven glial neoplasia in Drosophila (A) Optical projections of whole brain-nerve cord complexes from 3rd instar larvae approximately 130 hrs old. Dorsal view; anterior up. CD8-GFP (green) labels glial cell bodies. Compared to repo>dEGFRλ;dp110CAAX, warts knockdown (repo>wartsdsRNA; dEGFRλ;dp110CAAX) increased neoplastic brain overgrowth and yki knockdown (repo>ykidsRNA;dEGFRλ;dp110CAAX) decreased neoplastic brain overgrowth. (B) 3 µm optical projections of brain hemispheres, age-matched 3rd instar larvae. Frontal sections; anterior up; midline to left. Repo (red) labels glial cell nuclei; CD8-GFP (green) labels glial cell bodies; anti-HRP (blue) counter-stains for neurons and neuropil. (middle) repo>dEGFRλ;dp110CAAX showed increased glial cell numbers (red nuclei) compared to (upper left) wild-type. Compared to repo>dEGFRλ;dp110CAAX, (right) warts knockdown increased neoplastic glial cell numbers (red nuclei), whereas (lower left) yki knockdown reduced neoplastic glial cell numbers (red nuclei). (C, D) Low levels of Yki protein (red) was observed in wild-type central brain glia (white arrows, left panel in C) compared to high levels of cytoplasmic and nuclear Yki protein in dEGFRλ;dp110CAAX neoplastic glia (white arrows, left panel in D); Repo (blue) labels glial cell nuclei; CD8-GFP (green) labels glial cell bodies. Vigneswaran et al Supplementary Data Figure S2: YAP/TAZ expression confined to RTK-amplified tuMor cells and Maintained in patient-derived xenografts (A) On the left, immunohistochemical (IHC) staining in representative normal brain parenchyma in the cortex where YAP expression and TAZ expression was limited to vascular cells and was not detectable in normal neuronal and glial cells. -

U1 Snrnp Regulates Chromatin Retention of Noncoding Rnas
bioRxiv preprint doi: https://doi.org/10.1101/310433; this version posted April 29, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. U1 snRNP regulates chromatin retention of noncoding RNAs Yafei Yin,1,* J. Yuyang Lu,1 Xuechun Zhang,1 Wen Shao,1 Yanhui Xu,1 Pan Li,1,2 Yantao Hong,1 Qiangfeng Cliff Zhang,2 and Xiaohua Shen 1,3,* 1 Tsinghua-Peking Center for Life Sciences, School of Medicine and School of Life Sciences, Tsinghua University, Beijing 100084, China 2 MOE Key Laboratory of Bioinformatics, Beijing Advanced Innovation Center for Structural Biology, Center for Synthetic and Systems Biology, School of Life Sciences, Tsinghua University, Beijing 100084, China 3 Lead Contact *Correspondence: [email protected] (Y.Y.), [email protected] (X.S.) Running title: U1 snRNP regulates ncRNA-chromatin retention Keywords: RNA-chromatin localization, U1 snRNA, lncRNA, PROMPTs and eRNA, splicing 1 bioRxiv preprint doi: https://doi.org/10.1101/310433; this version posted April 29, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Thousands of noncoding transcripts exist in mammalian genomes, and they preferentially localize to chromatin. Here, to identify cis-regulatory elements that control RNA-chromatin association, we developed a high-throughput method named RNA element for subcellular localization by sequencing (REL-seq). Coupling REL-seq with random mutagenesis (mutREL-seq), we discovered a key 7-nt U1 recognition motif in chromatin-enriched RNA elements. -

RNA Splicing Regulated by RBFOX1 Is Essential for Cardiac Function in Zebrafish Karen S
© 2015. Published by The Company of Biologists Ltd | Journal of Cell Science (2015) 128, 3030-3040 doi:10.1242/jcs.166850 RESEARCH ARTICLE RNA splicing regulated by RBFOX1 is essential for cardiac function in zebrafish Karen S. Frese1,2,*, Benjamin Meder1,2,*, Andreas Keller3,4, Steffen Just5, Jan Haas1,2, Britta Vogel1,2, Simon Fischer1,2, Christina Backes4, Mark Matzas6, Doreen Köhler1, Vladimir Benes7, Hugo A. Katus1,2 and Wolfgang Rottbauer5,‡ ABSTRACT U2, U4, U5 and U6) and several hundred associated regulator Alternative splicing is one of the major mechanisms through which proteins (Wahl et al., 2009). Some of the best-characterized the proteomic and functional diversity of eukaryotes is achieved. splicing regulators include the serine-arginine (SR)-rich family, However, the complex nature of the splicing machinery, its associated heterogeneous nuclear ribonucleoproteins (hnRNPs) proteins, and splicing regulators and the functional implications of alternatively the Nova1 and Nova2, and the PTB and nPTB (also known as spliced transcripts are only poorly understood. Here, we investigated PTBP1 and PTBP2) families (David and Manley, 2008; Gabut et al., the functional role of the splicing regulator rbfox1 in vivo using the 2008; Li et al., 2007; Matlin et al., 2005). The diversity in splicing is zebrafish as a model system. We found that loss of rbfox1 led to further increased by the location and nucleotide sequence of pre- progressive cardiac contractile dysfunction and heart failure. By using mRNA enhancer and silencer motifs that either promote or inhibit deep-transcriptome sequencing and quantitative real-time PCR, we splicing by the different regulators. Adding to this complexity, show that depletion of rbfox1 in zebrafish results in an altered isoform regulating motifs are very common throughout the genome, but are expression of several crucial target genes, such as actn3a and hug. -

U1 Snrnp Regulates Chromatin Retention of Noncoding Rnas
Article U1 snRNP regulates chromatin retention of noncoding RNAs https://doi.org/10.1038/s41586-020-2105-3 Yafei Yin1 ✉, J. Yuyang Lu1, Xuechun Zhang1, Wen Shao1, Yanhui Xu1, Pan Li1,2, Yantao Hong1, Li Cui1, Ge Shan3, Bin Tian4, Qiangfeng Cliff Zhang1,2 & Xiaohua Shen1 ✉ Received: 26 November 2018 Accepted: 9 January 2020 Long noncoding RNAs (lncRNAs) and promoter- or enhancer-associated unstable Published online: xx xx xxxx transcripts locate preferentially to chromatin, where some regulate chromatin structure, Check for updates transcription and RNA processing1–13. Although several RNA sequences responsible for nuclear localization have been identifed—such as repeats in the lncRNA Xist and Alu-like elements in long RNAs14–16—how lncRNAs as a class are enriched at chromatin remains unknown. Here we describe a random, mutagenesis-coupled, high-throughput method that we name ‘RNA elements for subcellular localization by sequencing’ (mutREL-seq). Using this method, we discovered an RNA motif that recognizes the U1 small nuclear ribonucleoprotein (snRNP) and is essential for the localization of reporter RNAs to chromatin. Across the genome, chromatin-bound lncRNAs are enriched with 5′ splice sites and depleted of 3′ splice sites, and exhibit high levels of U1 snRNA binding compared with cytoplasm-localized messenger RNAs. Acute depletion of U1 snRNA or of the U1 snRNP protein component SNRNP70 markedly reduces the chromatin association of hundreds of lncRNAs and unstable transcripts, without altering the overall transcription rate in cells. In addition, rapid degradation of SNRNP70 reduces the localization of both nascent and polyadenylated lncRNA transcripts to chromatin, and disrupts the nuclear and genome-wide localization of the lncRNA Malat1.