Evidence of a False Thumb in a Fossil Carnivore Clarifies the Evolution of Pandas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
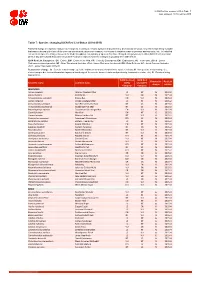
Table 7: Species Changing IUCN Red List Status (2014-2015)
IUCN Red List version 2015.4: Table 7 Last Updated: 19 November 2015 Table 7: Species changing IUCN Red List Status (2014-2015) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2014 (IUCN Red List version 2014.3) and 2015 (IUCN Red List version 2015-4) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2014) List (2015) change version Category Category MAMMALS Aonyx capensis African Clawless Otter LC NT N 2015-2 Ailurus fulgens Red Panda VU EN N 2015-4 -

Dental and Temporomandibular Joint Pathology of the Kit Fox (Vulpes Macrotis)
Author's Personal Copy J. Comp. Path. 2019, Vol. 167, 60e72 Available online at www.sciencedirect.com ScienceDirect www.elsevier.com/locate/jcpa DISEASE IN WILDLIFE OR EXOTIC SPECIES Dental and Temporomandibular Joint Pathology of the Kit Fox (Vulpes macrotis) N. Yanagisawa*, R. E. Wilson*, P. H. Kass† and F. J. M. Verstraete* *Department of Surgical and Radiological Sciences and † Department of Population Health and Reproduction, School of Veterinary Medicine, University of California, Davis, California, USA Summary Skull specimens from 836 kit foxes (Vulpes macrotis) were examined macroscopically according to predefined criteria; 559 specimens were included in this study. The study group consisted of 248 (44.4%) females, 267 (47.8%) males and 44 (7.9%) specimens of unknown sex; 128 (22.9%) skulls were from young adults and 431 (77.1%) were from adults. Of the 23,478 possible teeth, 21,883 teeth (93.2%) were present for examina- tion, 45 (1.9%) were absent congenitally, 405 (1.7%) were acquired losses and 1,145 (4.9%) were missing ar- tefactually. No persistent deciduous teeth were observed. Eight (0.04%) supernumerary teeth were found in seven (1.3%) specimens and 13 (0.06%) teeth from 12 (2.1%) specimens were malformed. Root number vari- ation was present in 20.3% (403/1,984) of the present maxillary and mandibular first premolar teeth. Eleven (2.0%) foxes had lesions consistent with enamel hypoplasia and 77 (13.8%) had fenestrations in the maxillary alveolar bone. Periodontitis and attrition/abrasion affected the majority of foxes (71.6% and 90.5%, respec- tively). -

Chapter 1 - Introduction
EURASIAN MIDDLE AND LATE MIOCENE HOMINOID PALEOBIOGEOGRAPHY AND THE GEOGRAPHIC ORIGINS OF THE HOMININAE by Mariam C. Nargolwalla A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Graduate Department of Anthropology University of Toronto © Copyright by M. Nargolwalla (2009) Eurasian Middle and Late Miocene Hominoid Paleobiogeography and the Geographic Origins of the Homininae Mariam C. Nargolwalla Doctor of Philosophy Department of Anthropology University of Toronto 2009 Abstract The origin and diversification of great apes and humans is among the most researched and debated series of events in the evolutionary history of the Primates. A fundamental part of understanding these events involves reconstructing paleoenvironmental and paleogeographic patterns in the Eurasian Miocene; a time period and geographic expanse rich in evidence of lineage origins and dispersals of numerous mammalian lineages, including apes. Traditionally, the geographic origin of the African ape and human lineage is considered to have occurred in Africa, however, an alternative hypothesis favouring a Eurasian origin has been proposed. This hypothesis suggests that that after an initial dispersal from Africa to Eurasia at ~17Ma and subsequent radiation from Spain to China, fossil apes disperse back to Africa at least once and found the African ape and human lineage in the late Miocene. The purpose of this study is to test the Eurasian origin hypothesis through the analysis of spatial and temporal patterns of distribution, in situ evolution, interprovincial and intercontinental dispersals of Eurasian terrestrial mammals in response to environmental factors. Using the NOW and Paleobiology databases, together with data collected through survey and excavation of middle and late Miocene vertebrate localities in Hungary and Romania, taphonomic bias and sampling completeness of Eurasian faunas are assessed. -

An Educator's Resource to Texas Mammal Skulls and Skins
E4H-014 11/17 An Educator’s Resource to Texas Mammal Skulls and Skins for use in 4-H Wildlife Programs and FFA Wildlife Career Development Events By, Denise Harmel-Garza Program Coordinator I, Texas A&M AgriLife Extension Service, 4-H Photographer and coauthor, Audrey Sepulveda M.Ed. Agricultural Leadership, Education and Communications, Texas A&M University College Station, Texas 2017 “A special thanks to the Biodiversity Research and Teaching Collections at Texas A&M University for providing access to their specimens.” Texas A&M AgriLife Extension provides equal opportunities in its programs and employment to all persons, regardless of race, color, sex, religion, national origin, disability, age, genetic information, veteran status, sexual orientation, or gender identity. The Texas A&M University System, U.S. Department of Agriculture, and the County Commissioners Courts of Texas Cooperating. Introduction Texas youth that participate in wildlife programs may be asked to identify a skull, skin, scat, tracks, etc. of an animal. Usually, educators must find this information and assemble pictures of skulls and skins from various sources. They also must ensure that what they find is relevant and accurate. Buying skulls and skins to represent all Texas mammals is costly. Most educators cannot afford them, and if they can, maintaining these collections over time is problematic. This study resource will reduce the time teachers across the state need to spend searching for information and allow them more time for presenting the material to their students. This identification guide gives teachers and students easy access to information that is accurate and valuable for learning to identify Texas mammals. -

Musteloidea (Carnivora, Mammalia)
e390-11 Montoya.qxd 1/2/12 09:36 Página 193 Estudios Geológicos, 67(2) julio-diciembre 2011, 193-206 ISSN: 0367-0449 doi:10.3989/egeol.40576.183 Musteloidea (Carnivora, Mammalia) from the Late Miocene of Venta del Moro (Valencia, Spain) Musteloidea (Carnivora, Mammalia) del Mioceno Superior de Venta del Moro (Valencia, España) P.Montoya1, J. Morales2, J. Abella2 ABSTRACT The purpose of the present work is to describe the Musteloidea from the Late Miocene locality of Venta del Moro (Valencia, Spain). We have identified the following species: Martes ginsburgi nov. sp., Lutra affinis Gervais, 1859, Plesiogulo monspessulanus Viret, 1939 and Promephitis alexejewi Schloss- er, 1924. Besides Plesiogulo monspessulanus which was already described in this locality and in Las Casiones (MN 13, Teruel Basin), we are approaching an unedited Musteloidea assemblage from the Miocene of the Iberian Peninsula. The m1 of Martes ginsburgi nov. sp. is similar in size and morphology to the Asian species of the genus, M. anderssoni and M. zdanskyi, but it differs in having a very wide M1 with a developed lingual platform. The presence of Lutra affinis is the third register of this species in the fossil record, since it was only previously known from the Pliocene of Montpellier (France) and the termi- nal Miocene of Maramena (Greece). Promephitis alexejewi is the first appearance of this species in Europe, which has been only registered until now in several localities of Mongolia and China. It differs from the rest of Promephitis species in the possession of a narrower m1, with a very sectorial trigonid. Keywords: Mammalia, Carnivora, Musteloidea, Martes, Lutra, Plesiogulo, Promephitis, Venta del Moro, Spain, Upper Miocene RESUMEN Se describen los Musteloidea procedentes del Mioceno terminal (MN13) de Venta del Moro (Valencia, España). -

University of Florida Thesis Or Dissertation Formatting
UNDERSTANDING CARNIVORAN ECOMORPHOLOGY THROUGH DEEP TIME, WITH A CASE STUDY DURING THE CAT-GAP OF FLORIDA By SHARON ELIZABETH HOLTE A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2018 © 2018 Sharon Elizabeth Holte To Dr. Larry, thank you ACKNOWLEDGMENTS I would like to thank my family for encouraging me to pursue my interests. They have always believed in me and never doubted that I would reach my goals. I am eternally grateful to my mentors, Dr. Jim Mead and the late Dr. Larry Agenbroad, who have shaped me as a paleontologist and have provided me to the strength and knowledge to continue to grow as a scientist. I would like to thank my colleagues from the Florida Museum of Natural History who provided insight and open discussion on my research. In particular, I would like to thank Dr. Aldo Rincon for his help in researching procyonids. I am so grateful to Dr. Anne-Claire Fabre; without her understanding of R and knowledge of 3D morphometrics this project would have been an immense struggle. I would also to thank Rachel Short for the late-night work sessions and discussions. I am extremely grateful to my advisor Dr. David Steadman for his comments, feedback, and guidance through my time here at the University of Florida. I also thank my committee, Dr. Bruce MacFadden, Dr. Jon Bloch, Dr. Elizabeth Screaton, for their feedback and encouragement. I am grateful to the geosciences department at East Tennessee State University, the American Museum of Natural History, and the Museum of Comparative Zoology at Harvard for the loans of specimens. -

On the Socio-Sexual Behaviour of the Extinct Ursid Indarctos Arctoides: an Approach Based on Its Baculum Size and Morphology
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Digital.CSIC On the Socio-Sexual Behaviour of the Extinct Ursid Indarctos arctoides: An Approach Based on Its Baculum Size and Morphology Juan Abella1,2*, Alberto Valenciano3,4, Alejandro Pe´rez-Ramos5, Plinio Montoya6, Jorge Morales2 1 Institut Catala` de Paleontologia Miquel Crusafont, Universitat Auto`noma de Barcelona. Edifici ICP, Campus de la UAB s/n, Barcelona, Spain, 2 Museo Nacional de Ciencias Naturales-CSIC, Madrid, Spain, 3 Departamento de Geologı´a Sedimentaria y Cambio Medioambiental. Instituto de Geociencias (CSIC, UCM), Madrid, Spain, 4 Departamento de Paleontologı´a, Facultad de Ciencias Geolo´gicas UCM, Madrid, Spain, 5 Institut Cavanilles de Biodiversitat i Biologia Evolutiva, Universitat de Vale`ncia, Paterna, Spain, 6 Departament de Geologia, A` rea de Paleontologia, Universitat de Vale`ncia, Burjassot, Spain Abstract The fossil bacula, or os penis, constitutes a rare subject of study due to its scarcity in the fossil record. In the present paper we describe five bacula attributed to the bear Indarctos arctoides Depe´ret, 1895 from the Batallones-3 site (Madrid Basin, Spain). Both the length and morphology of this fossil bacula enabled us to make interpretative approaches to a series of ecological and ethological characters of this bear. Thus, we suggest that I. arctoides could have had prolonged periods of intromission and/or maintenance of intromission during the post-ejaculatory intervals, a multi-male mating system and large home range sizes and/or lower population density. Its size might also have helped females to choose from among the available males. -

Brain-Size Evolution and Sociality in Carnivora
Brain-size evolution and sociality in Carnivora John A. Finarellia,b,1 and John J. Flynnc aDepartment of Geological Sciences, University of Michigan, 2534 C.C. Little Building, 1100 North University Avenue, Ann Arbor, MI 48109; bMuseum of Paleontology, University of Michigan, 1529 Ruthven Museum, 1109 Geddes Road, Ann Arbor, MI 48109; and cDivision of Paleontology and Richard Gilder Graduate School, American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024 Edited by Alan Walker, Pennsylvania State University, University Park, PA, and approved April 22, 2009 (received for review February 16, 2009) Increased encephalization, or larger brain volume relative to body develop a comprehensive view of the evolutionary history of mass, is a repeated theme in vertebrate evolution. Here we present encephalization across 289 terrestrial species (including 125 an extensive sampling of relative brain sizes in fossil and extant extinct species) of Carnivora, providing an extensive sampling of taxa in the mammalian order Carnivora (cats, dogs, bears, weasels, fossil and living taxa for both major subclades: Caniformia and and their relatives). By using Akaike Information Criterion model Feliformia. selection and endocranial volume and body mass data for 289 species (including 125 fossil taxa), we document clade-specific Results evolutionary transformations in encephalization allometries. Akaike Information Criterion (AIC) model selection recovered These evolutionary transformations include multiple independent 4 optimal models (OM) within 2 log-likelihood units of the encephalization increases and decreases in addition to a remark- highest score (Table 1). There is broad agreement among the ably static basal Carnivora allometry that characterizes much of the OM with differences primarily in estimates of allometric slopes. -

References: Future Works
Phylogenomics and Evolution of the Ursidae Family Department of Biology Ammary Jackson, Keanu Spencer, & Alissya Theis Fig 8. Red Panda Fig. 6. American Black Bear (Ailurus fulgens) (Ursus americanus) Introduction: Ursidae is a family of generally omnivorous mammals colloquially Objectives: Results: referred to as bears. The family consists of five genera: Ailuropoda ● To determine the relatedness among the 30 individual bear taxa. Red Panda (giant panda), Helarctos (sun bear), Melursus (sloth bear), Tremarctos Spectacled Bear ● To determine if Ailurus fulgens obtained its common Spectacled Bear (spectacled bear), and Ursus (black, brown, and polar bears) all of Polar Bear name (Red Panda) from similarities to the genes Polar Bear which are found in North and South America, Europe, Asia, and Africa Polar Bear belonging to the Ursidae family or if it’s simply based on Polar Bear (Kumar et al. 2017.) The phylogenetic relationship between Ursidae Polar Bear phenotypic attributes. Polar Bear bears and the red panda (Ailurus fulgens) has been somewhat Brown Bear inconsistent and controversial. Previous phylogenetic analyses have Brown Bear Brown Bear placed the red panda within the families Ursidae (bears), Procyonidae Polar Bear Brown Bear (raccoons), Pinnepedia (seals), and Musteloidea (raccoons and weasels, Brown Bear Brown Bear skunks, and badgers) (Flynn et al. 2000.) Determining monophyly Methods: Cave Bear Cave Bear would elucidate the evolutionary relationship between Ursidae bears Sloth Bear ● Mitochondrial gene sequences of the ATP6 and ND1 genes Sloth Bear and the Red Panda. This analysis (i) tested the monophyly of the family Sun Bear were taken from a sample of 31 species (30 Ursidae family Sun Bear Ursidae; and (ii) determined how the Red Panda fits within the Black Bear and 1 Ailuridae family). -

Evolutionary History of Carnivora (Mammalia, Laurasiatheria) Inferred
bioRxiv preprint doi: https://doi.org/10.1101/2020.10.05.326090; this version posted October 5, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. This article is a US Government work. It is not subject to copyright under 17 USC 105 and is also made available for use under a CC0 license. 1 Manuscript for review in PLOS One 2 3 Evolutionary history of Carnivora (Mammalia, Laurasiatheria) inferred 4 from mitochondrial genomes 5 6 Alexandre Hassanin1*, Géraldine Véron1, Anne Ropiquet2, Bettine Jansen van Vuuren3, 7 Alexis Lécu4, Steven M. Goodman5, Jibran Haider1,6,7, Trung Thanh Nguyen1 8 9 1 Institut de Systématique, Évolution, Biodiversité (ISYEB), Sorbonne Université, 10 MNHN, CNRS, EPHE, UA, Paris. 11 12 2 Department of Natural Sciences, Faculty of Science and Technology, Middlesex University, 13 United Kingdom. 14 15 3 Centre for Ecological Genomics and Wildlife Conservation, Department of Zoology, 16 University of Johannesburg, South Africa. 17 18 4 Parc zoologique de Paris, Muséum national d’Histoire naturelle, Paris. 19 20 5 Field Museum of Natural History, Chicago, IL, USA. 21 22 6 Department of Wildlife Management, Pir Mehr Ali Shah, Arid Agriculture University 23 Rawalpindi, Pakistan. 24 25 7 Forest Parks & Wildlife Department Gilgit-Baltistan, Pakistan. 26 27 28 * Corresponding author. E-mail address: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2020.10.05.326090; this version posted October 5, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. This article is a US Government work. -

H420/02 Biological Diversity
A Level Biology A H420/02 Biological Diversity Question Set 11 1 The cheetah, Acinonyx jubatus, is a member of the cat family, Felidae. Cheetahs display less intraspecific variation than other members of the family Felidae. Fig. 20.1 shows the mean body length of a population of cheetahs from southern Africa. The error bars on Fig. 20.1 show the standard deviation of mean body length. Fig. 20.1 (a) (i) At between 2.5 and 4 years old, the mean length of female cheetahs is less than that of males. Calculate how much shorter than males female cheetahs are. Show your working. Express your answer as a percentage to two significant figures. Answer……………% [2] (ii) Using only Fig. 20.1 and your answer to (i), what can be concluded about the significance of the difference between the length of male and female cheetahs aged between 2.5 and 4 years? Explain your answer. [2] (iii) A student looked at Fig. 20.1 and wrote: “The longest male cheetah that was measured was 1.52 m long”. Explain whether the information in Fig. 20.1 supports the student’s answer. [1] (iv) State the likely causes of variation in body length in cheetahs. [2] (b) The population of cheetahs has been declining for the past 100 years and is estimated to be between 6000 and 7000. Within the remaining cheetah population, intraspecific genetic diversity is very low. One isolated population of cheetahs in Iran has fewer than 100 individuals. (i) State one way in which genetic diversity can be measured. -

Phylogeny of the Procyonidae (Mammalia: Carnivora): Molecules, Morphology and the Great American Interchange
Molecular Phylogenetics and Evolution 43 (2007) 1076–1095 www.elsevier.com/locate/ympev Phylogeny of the Procyonidae (Mammalia: Carnivora): Molecules, morphology and the Great American Interchange a, b c a Klaus-Peter KoepXi ¤, Matthew E. Gompper , Eduardo Eizirik , Cheuk-Chung Ho , Leif Linden a, Jesus E. Maldonado d, Robert K. Wayne a a Department of Ecology and Evolutionary Biology, University of California, Los Angeles, CA 90095-1606, USA b Department of Fisheries and Wildlife Sciences, University of Missouri, Colombia, MO 65211, USA c Faculdade de Biociencias, PUCRS, Av. Ipiranga, 6681, Predio 12, Porto Alegre, RS 90619-900, Brazil d Smithsonian Institution, NMNH/NZP—Genetic Program, 3001 Connecticut Avenue NW, Washington, DC 20008, USA Received 10 June 2006; revised 22 September 2006; accepted 2 October 2006 Available online 11 October 2006 Abstract The Procyonidae (Mammalia: Carnivora) have played a central role in resolving the controversial systematics of the giant and red pandas, but phylogenetic relationships of species within the family itself have received much less attention. Cladistic analyses of morpho- logical characters conducted during the last two decades have resulted in topologies that group ecologically and morphologically similar taxa together. SpeciWcally, the highly arboreal and frugivorous kinkajou (Potos Xavus) and olingos (Bassaricyon) deWne one clade, whereas the more terrestrial and omnivorous coatis (Nasua), raccoons (Procyon), and ringtails (Bassariscus) deWne another clade, with the similar-sized Nasua and Procyon joined as sister taxa in this latter group. These relationships, however, have not been tested with molecu- lar sequence data. We examined procyonid phylogenetics based on combined data from nine nuclear and two mitochondrial gene seg- ments totaling 6534 bp.