060125 Lansbergen-GJA.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Titel Interpret
Titel Interpret 15 MILJOEN MENSEN FLUITSMA & VAN THIJN 1948 GERARD COX 24 ROZEN TOON HERMANS AAN DIE AMSTERDAMSE GRACHTEN WIM ZONNEVELD AAN DE KUST BLOF ADEM MIJN ADEM PETER SSHAAP ADEMNOOD LINDA, ROOS & JESSICA ALLE DUIVEN OP DE DAM GERT & HERMIEN ALS STERREN STRALEN MARIANNE WEBER ANNABEL HANS DE BOOIJ ANNE HERMAN VAN VEEN ANTON AUS TIROL DE ZWARE JONGENS BANGER HART ROB DE NIJS BEESTJES RONNIE EN DE RONNIES BEN IK TE MIN ARMAND BIG CITY TOL HANSEN BIJ ONS STAAT OP DE KEUKENDEUR DE TWEE PINTEN BIJNA EDDY ZOEY BINNEN MARCO BORSATO BLIJF BIJ MIJ RUTH JACOTT BRABANTSE NACHTEN ZIJN LANG ARIE RIBBENS COMMENT CA VA THE SHORTS DAAR GAAT ZE CLOUSEAU DANS JE DE HELE NACHT MET MIJ DE JONNIES DAT AFGEZAAGDE ZINNETJE WILLY & WILLEKE ALBERTI DE BOM DOE MAAR DE CLOWN BEN CRAMER DE HEILSOLDAAT ZANGERES ZONDER NAAM ROZEN NAAR SANDRA RONNY TOBER DE NOZEM EN DE NON CORNELIS VREESWIJK DE OUDE MUZIKANT VADER ABRAHAM DE REGENBOOG FRANS BAUER & MARIANNE WEBER DE TWEE MOTTEN DORUS DE VEERPONT DRS. P DE VERZONKEN STAD FRANK & MIRELLA DE WAARHEID MARCO BORSATO DE WANDELCLUB SUGAR LEE HOOPER DE WILDE BOERNDOCHTERE IVAN HEYLEN DE ZEE TRIJNTJE OOSTERHUIS DIE LAAIELICHTER GERARD COX DIKKERTJE DAP HERMAN VAN VEEN DODENRIT DRS. P DROMEN ZIJN BEDROG MARCO BORSATO EEN PIKKETANUSSIE JOHNNY JORDAAN EEN ROOSJE M'N ROOSJE CONNY VANDENBOS FREKIE JOOST PRINSEN GEEF M'N HOOP JOMANDA GIJP GEWOON EEN VROLIJK LIEDJE DENNIS GOEDE TIJDEN SLECHTE TIJDEN LISA BORAY GROETEN UIT MAAIVELD ACDA & DE MUNNIK GUUS ALEXANDER CURLY HEB JE EVEN VOOR MIJ FRANS BAUER HET DORP WIM SONNEVELD -

March 5, 2009 the Free-Content News Source That You Can Write! Page 1
March 5, 2009 The free-content news source that you can write! Page 1 Top Stories Wikipedia Current Events Wikipedia Current Events International Criminal Court A riot at a prison near Ciudad escapes a Somali pirate attack in in The Hague issues arrest Juárez, Mexico, kills at least 20 the Gulf of Aden. warrant for leader of Sudan inmates and injures seven •The Netherlands' Safety Board The International Criminal Court others. finds that Turkish Airlines Flight (ICC) in The Hague, Netherlands •Gordon Brown becomes the 1951 crash-landed near has ordered the arrest of the United Kingdom's fifth Prime Amsterdam's Schiphol Airport president of the African country Minister to address a joint because of a faulty altimeter. of Sudan, Omar Hassan al-Bashir. session of the United States The warrant was issued after the Congress. ICC found al-Bashir guilty of Marine jet crash into San Diego seven charges of crimes against •President Nicolas Sarkozy and house blamed on string of humanity and war crimes, in the eight other top French politicians errors Darfur region of the country. receive death threats. An internal investigation by the United States Marine Corps into •China plans to increase its Two-time Eurovision entrant the crash of an F/A-18 jet into a military budget by 14.9% in Edsilia Rombley discusses San Diego house has blamed 2009. music, love, and her errors on the part of the pilot, his contrasting Contest •U.S. Secretary of State Hillary superiors and maintenance experiences Clinton and Palestinian National personnel for the accident which Edsilia Rombley's career Authority President Mahmoud killed four people. -

Radio 2 Paradeplaat Last Change Artiest: Titel: 1977 1 Elvis Presley
Radio 2 Paradeplaat last change artiest: titel: 1977 1 Elvis Presley Moody Blue 10 2 David Dundas Jeans On 15 3 Chicago Wishing You Were Here 13 4 Julie Covington Don't Cry For Me Argentina 1 5 Abba Knowing Me Knowing You 2 6 Gerard Lenorman Voici Les Cles 51 7 Mary MacGregor Torn Between Two Lovers 11 8 Rockaway Boulevard Boogie Man 10 9 Gary Glitter It Takes All Night Long 19 10 Paul Nicholas If You Were The Only Girl In The World 51 11 Racing Cars They Shoot Horses Don't they 21 12 Mr. Big Romeo 24 13 Dream Express A Million In 1,2,3, 51 14 Stevie Wonder Sir Duke 19 15 Champagne Oh Me Oh My Goodbye 2 16 10CC Good Morning Judge 12 17 Glen Campbell Southern Nights 15 18 Andrew Gold Lonely Boy 28 43 Carly Simon Nobody Does It Better 51 44 Patsy Gallant From New York to L.A. 15 45 Frankie Miller Be Good To Yourself 51 46 Mistral Jamie 7 47 Steve Miller Band Swingtown 51 48 Sheila & Black Devotion Singin' In the Rain 4 49 Jonathan Richman & The Modern Lovers Egyptian Reggae 2 1978 11 Chaplin Band Let's Have A Party 19 1979 47 Paul McCartney Wonderful Christmas time 16 1984 24 Fox the Fox Precious little diamond 11 52 Stevie Wonder Don't drive drunk 20 1993 24 Stef Bos & Johannes Kerkorrel Awuwa 51 25 Michael Jackson Will you be there 3 26 The Jungle Book Groove The Jungle Book Groove 51 27 Juan Luis Guerra Frio, frio 51 28 Bis Angeline 51 31 Gloria Estefan Mi tierra 27 32 Mariah Carey Dreamlover 9 33 Willeke Alberti Het wijnfeest 24 34 Gordon t Is zo weer voorbij 15 35 Oleta Adams Window of hope 13 36 BZN Desanya 15 37 Aretha Franklin Like -

Soul Top 1000
UUR 1: 14 april 9 uur JAAP 1000 Isley Brothers It’s Your Thing 999 Jacksons Enjoy Yourself 998 Eric Benet & Faith Evans Georgy Porgy 997 Delfonics Ready Or Not Here I Come 996 Janet Jackson What Have Your Done For Me Lately 995 Michelle David & The Gospel Sessions Love 994 Temptations Ain’t Too Proud To Beg 993 Alain Clark Blow Me Away 992 Patti Labelle & Michael McDonald On My Own 991 King Floyd Groove Me 990 Bill Withers Soul Shadows UUR 2: 14 april 10 uur NON-STOP 989 Michael Kiwanuka & Tom Misch Money 988 Gloria Jones Tainted Love 987 Toni Braxton He Wasn’t Man Enough 986 John Legend & The Roots Our Generation 985 Sister Sledge All American Girls 984 Jamiroquai Alright 983 Carl Carlton She’s A Bad Mama Jama 982 Sharon Jones & The Dap-Kings Better Things 981 Anita Baker You’re My Everything 980 Jon Batiste I Need You 979 Kool & The Gang Let’s Go Dancing 978 Lizz Wright My Heart 977 Bran van 3000 Astounded 976 Johnnie Taylor What About My Love UUR 3: 14 april 11 uur NON-STOP 975 Des’ree You Gotta Be 974 Craig David Fill Me In 973 Linda Lyndell What A Man 972 Giovanca How Does It Feel 971 Alexander O’ Neal Criticize 970 Marcus King Band Homesick 969 Joss Stone Don’t Cha Wanna Ride 1 968 Candi Staton He Called Me Baby 967 Jamiroquai Seven Days In Sunny June 966 D’Angelo Sugar Daddy 965 Bill Withers In The Name Of Love 964 Michael Kiwanuka One More Night 963 India Arie Can I Walk With You UUR 4: 14 april 12 uur NON-STOP 962 Anthony Hamilton Woo 961 Etta James Tell Mama 960 Erykah Badu Apple Tree 959 Stevie Wonder My Cherie Amour 958 DJ Shadow This Time (I’m Gonna Try It My Way) 957 Alicia Keys A Woman’s Worth 956 Billy Ocean Nights (Feel Like Gettin' Down) 955 Aretha Franklin One Step Ahead 954 Will Smith Men In Black 953 Ray Charles Hallelujah I Love Her So 952 John Legend This Time 951 Blu Cantrell Hit' m Up Style 950 Johnny Pate Shaft In Africa 949 Mary J. -
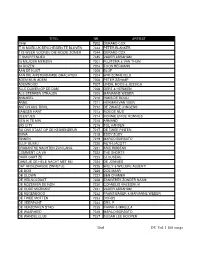
Karaoke Nummers NED-1 (Pdf)
TITEL NR. ARTIEST 1948 7202 GERARD COX 'T IS MOEILIJK BESCHEIDEN TE BLIJVEN 7343 PETER BLANKER T IS WEER VOORBIJ DIE MOOIE ZOMER 7344 GERARD COX 'T SMURFENLIED 7345 VADER ABRAHAM 15 MILJOEN MENSEN 7201 FLUITSMA & VAN THIJN 24 ROZEN 7203 TOON HERMANS AAN DE KUST 7205 BLOF AAN DIE AMSTERDAMSE GRACHTEN 7204 WIM ZONNEVELD ADEM MIJN ADEM 7206 PETER SSHAAP ADEMNOOD 7207 LINDA, ROOS & JESSICA ALLE DUIVEN OP DE DAM 7208 GERT & HERMIEN ALS STERREN STRALEN 7209 MARIANNE WEBER ANNABEL 7210 HANS DE BOOIJ ANNE 7211 HERMAN VAN VEEN ANTON AUS TIROL 7212 DE ZWARE JONGENS BANGER HART 7213 ROB DE NIJS BEESTJES 7214 RONNIE EN DE RONNIES BEN IK TE MIN 7215 ARMAND BIG CITY 7216 TOL HANSEN BIJ ONS STAAT OP DE KEUKENDEUR 7217 DE TWEE PINTEN BIJNA 7218 EDDY ZOEY BINNEN 7219 MARCO BORSATO BLIJF BIJ MIJ 7220 RUTH JACOTT BRABANTSE NACHTEN ZIJN LANG 7221 ARIE RIBBENS COMMENT CA VA 7222 THE SHORTS DAAR GAAT ZE 7223 CLOUSEAU DANS JE DE HELE NACHT MET MIJ 7224 DE JONNIES DAT AFGEZAAGDE ZINNETJE 7225 WILLY & WILLEKE ALBERTI DE BOM 7226 DOE MAAR DE CLOWN 7227 BEN CRAMER DE HEILSOLDAAT 7228 ZANGERES ZONDER NAAM DE NOZEM EN DE NON 7230 CORNELIS VREESWIJK DE OUDE MUZIKANT 7231 VADER ABRAHAM DE REGENBOOG 7232 FRANS BAUER & MARIANNE WEBER DE TWEE MOTTEN 7233 DORUS DE VEERPONT 7234 DRS. P DE VERZONKEN STAD 7235 FRANK & MIRELLA DE WAARHEID 7236 MARCO BORSATO DE WANDELCLUB 7237 SUGAR LEE HOOPER Titel DU Vol.1 166 songs TITEL NR. ARTIEST DE WILDE BOERNDOCHTERE 7238 IVAN HEYLEN DE ZEE 7239 TRIJNTJE OOSTERHUIS DIE LAAIELICHTER 7240 GERARD COX DIKKERTJE DAP 7241 HERMAN VAN VEEN DODENRIT 7242 DRS. -

60Th Eurovision Song Contest 2015 Vienna / Austria 1St Semi Final 19Th May 2015
60th Eurovision Song Contest 2015 Vienna / Austria 1st Semi Final 19th May 2015 Country Participant Song My Notes My Points Rank Qualifer 1 Moldova Eduard Romanyuta I Want Your Love 2 Armenia Genealogy Face The Shadow 3 Belgium Loïc Nottet Rhythm Inside 4 Netherlands Trijntje Oosterhuis Walk Along 5 Finland Pertti Kurikan Nimipäivät Aina Mun Pitää 6 Greece Maria-Elena Kyriakou Last Breath 7 Estonia Elina Born & Stig Rästa Goodbye To Yesterday 8 FYR Macedonia Daniel Kajmakoski Autumn Leaves 9 Serbia Bojana Stamenov Beauty Never Lies 10 Hungary Boggie Wars For Nothing 11 Belarus Uzari & Maimuna Time 12 Russia Polina Gagarina A Million Voices 13 Denmark Anti Social Media The Way You Are 14 Albania Elhaida Dani I'm Alive 15 Romania Voltaj De La Capăt 16 Georgia Nina Sublati Warrior [email protected] http://www.eurovisionlive.com © eurovisionlive.com 60th Eurovision Song Contest 2015 Vienna / Austria 2nd Semi Final 21st May 2015 Country Participant Song My Notes My Points Rank Qualifier 1 Lithuania Monika & Vaidas This Time 2 Ireland Molly Sterling Playing With Numbers 3 San Marino Michele Perniola & Anita Simoncini Chain Of Lights 4 Montenegro Knez Adio 5 Malta Amber Warrior 6 N0rway Mørland & Debrah Scarlett A Monster Like Me 7 Portugal Leonor Andrade Há Um Mar Que Nos Separa 8 Czech Republic Marta Jandová & Václav Noid Bárta Hope Never Dies 9 Israel Nadav Guedj Golden Boy 10 Latvia Aminata Love Injected 11 Azerbaijan Elnur Huseynov Hour Of The Wolf 12 Iceland María Ólafsdóttir Unbroken 13 Sweden Måns Zelmerlöw Heroes 14 Switzerland Mélanie René Time To Shine 15 Cyprus Giannis Karagiannis One Thing I Should Have Done 16 Slovenia Maraaya Here For You 17 Poland Monika Kuszyńska In The Name Of Love [email protected] http://www.eurovisionlive.com © eurovisionlive.com. -

Top 4000 2017
TOP 4000 2017 NR ARTIEST NUMMER JAAR 1 QUEEN BOHEMIAN RHAPSODY 1975 2 EAGLES HOTEL CALIFORNIA 1977 3 MICHAEL JACKSON THRILLER 1983 4 PRINCE PURPLE RAIN 1984 5 GUNS N' ROSES NOVEMBER RAIN 1992 6 PHIL COLLINS IN THE AIR TONIGHT 1981 7 U 2 ONE 1992 8 BILLY JOEL PIANO MAN 1974 9 LED ZEPPELIN STAIRWAY TO HEAVEN 1971 10 GEORGE MICHAEL CARELESS WHISPER 1984 11 MEATLOAF PARADISE BY THE DASHBOARDLIGHT 1978 12 BEATLES LET IT BE 1970 13 GOLDEN EARRING RADAR LOVE 1973 14 QUEEN INNUENDO 1991 15 DIRE STRAITS BROTHERS IN ARMS 1989 16 METALLICA ONE 1994 17 MICHAEL JACKSON BILLIE JEAN 1983 18 DEEP PURPLE CHILD IN TIME 1975 19 TOTO AFRICA 1982 20 JOHN MILES MUSIC 1976 21 METALLICA NOTHING ELSE MATTERS 1992 22 PINK FLOYD WISH YOU WERE HERE 1975 23 DAVID BOWIE LET'S DANCE 1983 24 DIRE STRAITS PRIVATE INVESTIGATIONS 1982 25 BOUDEWIJN DE GROOT AVOND 1997 26 ABBA DANCING QUEEN 1976 27 GUNS N' ROSES SWEET CHILD O'MINE 1988 28 PHARRELL WILLIAMS HAPPY 2013 29 EAGLES THE LAST RESORT 1976 30 MADONNA LIKE A PRAYER 1989 31 SUPERTRAMP SCHOOL 1974 32 FLEETWOOD MAC GO YOUR OWN WAY 1977 33 BON JOVI BED OF ROSES 1993 34 SIMON & GARFUNKEL THE SOUNDS OF SILENCE 1966 TOP 4000 4 t/m 24 december 2017 NR ARTIEST NUMMER JAAR 35 GEORGE MICHAEL & QUEEN SOMEBODY TO LOVE 1993 36 PEARL JAM ALIVE 1992 37 ELVIS PRESLEY SUSPICIOUS MINDS 1969 38 BRYAN ADAMS SUMMER OF '69 1990 39 U 2 WITH OR WITHOUT YOU 1987 40 BRUCE SPRINGSTEEN THE RIVER 1981 41 COLDPLAY FIX YOU 2005 42 MARILLION KAYLEIGH 1985 43 JOHN LENNON IMAGINE 1971 44 ROBBIE WILLIAMS ANGELS 1998 45 ELECTRIC LIGHT ORCHESTRA MR. -

Repertoire Patricia Foort
Repertoire Patricia Foort Soul/Motown: 1 Ain't got no (Nina Simone) 2 A song for you (Donny Hathaway) 3 Ain't no stopping us now(Luther Vandross) 4 Ain't no sunshine (Bill Withers of Jacksons) 5 Ain't nobody (Chaka Khan) 6 All in love is fair(Stevie Wonder) 7 All my life (K-ci and Jojo 8 All night long (Lionel Ritchie) 9 All of me (John Legend) 10 All the love in the world (Dionne Warwick) 11 Allways There (Incognito) 12 Almaz (Randy Crawford) 13 And I love you so (Perry Como) 14 Ave Maria(Beyonce) 15 Baby can I hold you tonight (Tracey Chapman) 16 Baby come to me (Patty Austin) 17 Baby I love you(Aretha Franklin) 18 Blueberry Hill (Fats Domino) 19 Bring it on home (Sam Cooke) 20 Chain of fool(Aretha Franklin) 21 Change is gonna come(Sam Cooke) 22 Clown (Emili Sande) 23 Dirty old man (The Three Degrees) 24 Do that to me (Captain and Tenille) 25 Don't let the sun go down (Oleta Adams) 26 Don't you worry 'bout a thing(Incognito) 27 Do you know where you're going to (Diana Ross) 28 Dr Feelgood (Aretha Franklin) 29 End of the road (Boys II Men) 30 Endlessly (Randy Crawford) 31 Fire (Pointer Sisters) 32 For all we know (Donny Hathaway) 33 For once in my life (Stevie Wonder/Trijntje) 34 Get here (Oleta Adams) 35 Go Like Elijah(Chi Coltrane) 36 Good Times (Chiq) 37 Hallelujah(Lisa Lois) 38 Happy (Pharrell Williams) 39 Happy Birthday (Stevie Wonder) 40 Hello (L. -

Karaoke Song Book Karaoke Nights Frankfurt’S #1 Karaoke
KARAOKE SONG BOOK KARAOKE NIGHTS FRANKFURT’S #1 KARAOKE SONGS BY TITLE THERE’S NO PARTY LIKE AN WAXY’S PARTY! Want to sing? Simply find a song and give it to our DJ or host! If the song isn’t in the book, just ask we may have it! We do get busy, so we may only be able to take 1 song! Sing, dance and be merry, but please take care of your belongings! Are you celebrating something? Let us know! Enjoying the party? Fancy trying out hosting or KJ (karaoke jockey)? Then speak to a member of our karaoke team. Most importantly grab a drink, be yourself and have fun! Contact [email protected] for any other information... YYOUOU AARERE THETHE GINGIN TOTO MY MY TONICTONIC A I L C S E P - S F - I S S H B I & R C - H S I P D S A - L B IRISH PUB A U - S R G E R S o'reilly's Englische Titel / English Songs 10CC 30H!3 & Ke$ha A Perfect Circle Donna Blah Blah Blah A Stranger Dreadlock Holiday My First Kiss Pet I'm Mandy 311 The Noose I'm Not In Love Beyond The Gray Sky A Tribe Called Quest Rubber Bullets 3Oh!3 & Katy Perry Can I Kick It Things We Do For Love Starstrukk A1 Wall Street Shuffle 3OH!3 & Ke$ha Caught In Middle 1910 Fruitgum Factory My First Kiss Caught In The Middle Simon Says 3T Everytime 1975 Anything Like A Rose Girls 4 Non Blondes Make It Good Robbers What's Up No More Sex.... -

TRIJNTJE OOSTERHUIS 2015 ,,Ik Doe Alleen Dingen Waar Ik Me Goed
TRIJNTJE OOSTERHUIS 2015 ,,Ik doe alleen dingen waar ik me goed bij voel en die me raken.” Het is het levensmotto van Trijntje Oosterhuis. De zangeres stapt nooit ergens in zonder er een goed gevoel bij te hebben. ,,Als ik volledig achter iets sta, kan de hele wereld instorten. Ik blijf ervoor gaan.” Die gedrevenheid en passie zorgt ervoor dat Trijntje al ruim 20 jaar aan de absolute top in Nederland staat. Maar hoe populair ook, ze zal nooit haar roots verloochenen.,,Ik voel mij het meest gelukkig op een ranzig podium met goede muzikanten en een slecht geluid. Daar komt mijn pure passie vandaan en het is de reden waarom ik nog steeds met zoveel plezier muziek maak. Ik wil steeds terug naar dat gevoel.” Nederland leerde Trijntje in 1996 kennen als boegbeeld van de groep Total Touch. ,,Total Touch heb ik opgericht met mijn broer Tjeerd. Het was een geweldige tijd.” Haar moed om in nieuwe projecten te duiken was er al vanaf het begin. ,,Er is nooit angst, zolang datgene wat ik doe ook klopt met wie ik ben. Soms voel ik mij wat vervreemd bij alle aandacht die ik krijg. Ik vind dat je aandacht moet verdienen.” En dat heeft ze de afgelopen 20 jaar met verve gedaan. ,,Ik ben zeer blij en dankbaar met alle projecten die ik heb omarmd. Ik ben als mens en daarmee als muzikant continu in ontwikkeling. Zolang ik er maar mijn hart als zangeres in kan leggen.” Die instelling en haar geloofwaardige performances, bracht Trijntje op zeer bijzondere plekken. Al op haar 24ste stond ze tijdens het fameuze North Sea Jazz Festival met jazzlegende Herbie Hancock op het podium. -

'Hoe Kun Je Nou Boos Worden Over Een Jurk?'
GROOT INTERVIEW TRIJNTJE OOSTERHUIS Na 25 jaar in het vak, scha- kelt Trijntje Oosterhuis (44) over naar haar moerstaal. Haar nieuwe theatershow wordt een ode aan het Ne- derlandse lied. ‘Nederlands- talige muziek stond al heel lang op mijn lijstje, maar de tijd was er simpelweg nog niet rijp voor.’ TEKST FR ANK WA ALS FOTOGRAFIE CORNÉ VAN DER STELT ‘Hoe kun je nou boos worden over een jurk?’ 44 — NIEUWE REVU NIEUWE REVU — 45 Mensen waren echt boos. ‘Dat verbaasde me nog het meest, die vele hefti- NIEUWE REVU ONTMOET ge reacties van kwade en verveelde mensen dat TRIJNTJE het teweegbracht. Hoe kun je nou boos worden OOSTERHUIS over een jurk? Gelukkig nam ik niets persoon- lijk en gleed het gemakkelijk van me af. Veel Waar? Kerkgebouw erger had ik het gevonden als men had gezegd De Duif in Amster- dat ik slecht zong, en dat het dan ook nog waar dam. zou zijn geweest. Dan had ik echt een probleem Wanneer? Op 30 gehad en was ik het liefst onder de tegels gekro- maart. ‘Als je aankomt met een jurk tot aan je klit, met pen om er de komende jaren niet meer onder Verder nog wat? Aeen soort uitsnede tot aan je schaambot, dan vandaan te komen. Raak je me namelijk met zin- Tijdens ons moet je niet verbaasd staan te kijken als daar gen, dan raak je me in het hart.’ gesprek hingen er wat opmerkingen over komen. En dan dat rouw- twee onderhouds- lapje dat ze afgooide. Toen ik het zag dacht ik Waar mensen ook over vielen is dat je medewerkers met echt even: sjonge, shit zeg! What the hell?’ tijdens het optreden vrij bedrukt in de gevaar voor eigen leven metershoog Klare taal van Anouk, schrijfster van Trijntje camera keek. -

Holland 3 Doors Down
257er - Holland 3 Doors Down - Kryptonite 3JS - Wiegelied Aaliyah - Rock the boat Aaliyah - Try again Abba - Chiquitita Abba - Dancing queen Abba - Does your mother know Abba - Eagle Abba - Fernando Abba - Gimme gimme gimme Abba - I have a dream Abba - Knowing me, knowing you Abba - Mamma mia Abba - Medley Abba - Super trouper Abba - Take a chance on me Abba - Thank you for the music Abba - The winner takes it all Abba - Waterloo Abel - Onderweg ACDC - Highway to hell Ace of Base - Don't turn around Ace of Base - The sign Ad Libs - The boy from New York City Addams Family - Theme song Adele - Chasing pavements 1 Adele - Fastlove Adele - Make you feel my love Adele - Rolling in the deep Adele - Rumour has it Adele - Set fire to the rain Adele - Skyfall Adele - Someone like you Adele - Turning table Aerosmith - I dont want to miss a thing Aerosmith - Janie's got a gun A-ha - Hunting high and low A-ha - Take on me Air Supply - All out of love Al Martino - Spanish eyes Aladdin - A whole new world Alan Walker - Alone Alan Walker - Faded Alanis Morissette - Ironic Alanis Morissette - You learn Alanis Morissette - You oughta know Alannah Myles - Black velvet Albert Hammond - It never rains in Southern California Alessia Cara - Scars to your beautiful Alesso - Heroes Alex - Een bossie rode rozen Alex de Meijer - Jij hoort bij mij Alexis Jordan - Happiness 2 Ali B - Ik huil alleen bij jou Alice Cooper - No more mister nice guy Alice Cooper - Poison Alicia Bridges - I love the nightlife Alicia Keys - A woman's worth Alicia Keys - Empire state of