The Effect of Cranial Cruciate Ligament Rupture on Range of Motion in Dogs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Medial Patellar Luxation
Allen L. Johnson, DVM, DACVS Alexander Z. Aguila, DVM, DACVS Russell L. Bennett, DVM, DACVS Kristin K. Shaw, DVM, PhD, DACVS, DACVSMR MEDIAL PATELLAR LUXATION WHAT IS MEDIAL PATELLAR LUXATION (MPL)? Patellar luxation is a condition in which the patella (kneecap) slips out of its normal location within the stifle (knee). It has been described as the most common congenital malformation in dogs, diagnosed in 7% of puppies, and can manifest in different types of luxation and varying grades of severity. In about half the cases, luxation occurs in both knees. Small-breed dogs are affected at 10 times the rate found in large breed dogs. In a normal stifle, the patella rides inside a groove located toward the bottom end of the femur (thigh bone). It is held in place by the patellar tendon, which attaches at the top to the quadriceps (thigh muscle) and at the bottom to Normal stifle (knee) in left image the upper end of the tibia (shin bone). The quadriceps, patella and patellar and stifle with luxation in right tendon are normally well-aligned and act to extend the lower leg. A dog with image. a patellar luxation generally has an abnormally shallow femoral groove and 1 – Patella in normal position there is a malformation of the system that extends the leg. 2 – Femur 3 – Patellar tendon This allows the patella to slip toward the inside of the stifle (medial patellar 4 – Tibia luxation) or toward the outside of the stifle (lateral patellar luxation). 5 – Luxated patella WHAT ARE THE COMMON SYMPTOMS OF MPL? The clinical signs of MPL vary greatly with the severity of the disease, ranging from non-symptomatic to complete lameness in the affected leg. -

Cruciate Ligament Disease in Dogs Advice Sheet
Cruciate Ligament Disease in Dogs Advice Sheet What is it? How is cranial cruciate ligament The canine stifle (knee) joint consists of disease diagnosed? an articulation between the femur (thigh Your dog will have an orthopaedic bone) and tibia (shin bone). There are two examination, which usually reveal signs of cruciate ligaments within the stifle joint a joint effusion (an increase in the amount called the cranial and caudal cruciate of fluid within the stifle), thickening of the ligaments which are fibrous bands that joint and muscle wastage. Manipulation provide stability to the joint during weight of the joint also allows the identification bearing and twisting movements, and of instability. Following orthopaedic resisting movement between the femur assessment, your dog will need to have and tibia during weight bearing. Cranial a sedation or short general anaesthetic cruciate ligament disease is a common for examination of the stifle joint to further condition that affects dogs. This ligament assess joint instability and for x-rays to be may degenerate with age, weaken and performed of the stifle joint, which can then then eventually rupture. Once stretched or be used to plan surgery. ruptured (torn) the stifle becomes painful and unstable for the dog. Sometimes we What are the treatment options for see traumatic ruptures, but in most dogs Cranial Cruciate Ligament Disease? the rupture occurs in a ligament with pre- existing degenerative changes, often Surgical Treatment following minimal trauma. Surgical treatment for cruciate rupture is We see complete or partial ruptures. Partial commonly recommended as it typically cruciate ruptures are unlikely to heal and offers a superior outcome. -

The Stifle Joint Is a Complex Joint in the Hind Limbs of Quadruped Animals. It Is the Equivalent Joint to the Human Knee
Rachel Watkins, Meadow Farm Hydrotherapy, North Common, Hepworth, Diss, IP22 2PR The stifle joint is a complex joint in the hind limbs of quadruped animals. It is the equivalent joint to the human knee. Although the primary movement is like a hinge, the menisci (cartilaginous spacers) allow a gliding action during movement so that the axis of rotation of the femur relative to the tibia varies according to the degree of flexion. Medial and lateral rotation of the tibia is also possible. The stifle consists of three interrelated joints; the femorotibial, the femoropatellar and the proximal tibiofibular. There are four sesamoid bones: the patella, medial and lateral fabellae and the popliteal sesamoid. These sesamoid bones assist with the smooth movement of a tendon/muscle over the joint. The most well- known sesamoid bone is the patella, more commonly known as the 'knee cap' and is associated with the patella ligament. It is located cranially to the joint and sits in the trochlear groove of the femur. The joint is stabilised by paired collateral ligaments (which act to prevent abduction and adduction of the joint) and paired cruciate ligaments. The cruciate and patella ligament are articular ligaments which join bone to bone at the stifle joint. These ligaments are not a single structure but are made up of a bundle of individual collagenous fibres and spindle-shaped cells known as fibrocytes, with little ground substance (an amorphous gel-like substance present in the composition of the various connective tissues). These fibres are tightly bound together to form the ligament. In a healthy ligament the fibres are all intact with no indication of inflammation or degeneration. -

Cruciate Ligament Tears
Cruciate Ligament Tears Anatomy The canine knee joint, known as the stifle joint, is similar to a human’s knee in many regards. The joint is made up of the femur (thigh bone), tibia (shin bone), and the patella (kneecap) that are firmly held together by ligaments. Ligaments are strong, dense structures consisting of connective tissue that join the ends of two bones across a joint. The function of ligaments is to stabilize the joint, like a hinge on a door. The stifle has two very important ligaments called the cranial (CrCL) and caudal (CaCL) cruciate ligaments (cruciate means a cross or crucifix) that cross in the center of the joint. The CrCL (known as the ACL in humans) restrains the backward and forward motion of the joint, in addition to inward twisting and hyperextension of the joint. CrCL is most commonly injured in dogs. In fact, more than 600,000 dogs in the U.S. have surgery for this problem every year. The stifle also has two half-moon shaped cartilage structures between the weight-bearing bone ends called the menisci. There are two menisci in each stifle, one on the inner side of the joint called the medial meniscus and one on the outer side of the joint called the lateral meniscus. The menisci add support to the stifle and also serve as shock absorbers by spreading the weight load. Effects of CrCL tear The top of the tibia bone, called the tibial plateau is angled downward. When the CCL is torn, weight-bearing forces causes the femur bone to slide down this slope. -
Patellar Luxation This Information Is Provided to Help You Understand the Condition That Has Been Diagnosed in Your Pet
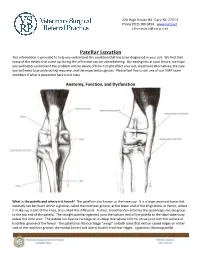
220 High House Rd. Cary NC 27513 Phone (919) 380-9494 www.vsrp.net [email protected] Patellar Luxation This information is provided to help you understand the condition that has been diagnosed in your pet. We find that many of the details that come up during the office visit can be overwhelming. By reading this at your leisure, we hope you will better understand the problem and be aware of how it might affect your pet, treatment alternatives, the care you will need to provide during recovery, and the expected prognosis. Please feel free to ask one of our VSRP team members if what is presented here is not clear. Anatomy, Function, and Dysfunction What is the patella and where is it found? The patella is also known as the knee cap. It is a large sesamoid bone that normally can be found within a groove, called the trochlear groove, at the lower end of the thigh bone, or femur, where it makes up a part of the knee, also called the stifle joint. A stout, broad tendon attaches the quadriceps muscle group to the top end of the patella. The straight patellar ligament joins the bottom end of the patella to the tibial tuberosity below the stifle joint. The patella has hyaline cartilage on its deep face where it forms a true joint with the surface of trochlear groove of the femur. The patella has fibrocartilage “wings” on both sides that rest on raised ridges on either side of the trochlear groove; the medial (inner) and lateral (outer) trochlear ridges. -

ANATOMICAL, RADIOLOGICAL and MAGNETIC RESONANCE IMAGING on the NORMAL STIFLE JOINT in RED FOX (VULPES VULPES) Samah
International Journal of Anatomy and Research, Int J Anat Res 2017, Vol 5(4.3):4760-69. ISSN 2321-4287 Original Research Article DOI: https://dx.doi.org/10.16965/ijar.2017.465 ANATOMICAL, RADIOLOGICAL AND MAGNETIC RESONANCE IMAGING ON THE NORMAL STIFLE JOINT IN RED FOX (VULPES VULPES) Samah. H. El-Bably *, Nawal. A. Noor Department of Anatomy & Embryology, Faculty of Veterinary Medicine, Cairo University, Egypt. ABSTRACT Background and Aim: Our study was an attempt to study the normal anatomy of stifle joint in the fox, that’s not recorded in any of available works of literature. Material and Methods: using dissection of the stifle, latex injection for its arterial supply as well as studying the normal Radiology and Magnetic Resonance Imaging Technique were adopted. The obtained results were photographed using Nikon digital camera 20 megapixels, 16X and discussed with their corresponding features of authors who performed earlier studies in other species, especially canine. Results: The articular surfaces, capsule, ligaments, and menisci of the stifle were described. The arterial blood supply of the joint also studied and mainly achieved through the descending genicular and popliteal arteries. Conclusion: this study was an attempt to help the anatomists in comparative studies and surgeons in surgical operations. KEY WORDS: Anatomy, Fox, Joints, Stifle. Address for Correspondence: Dr. Samah Elbably Assistant professor, Department of Anatomy, Faculty of Veterinary Medicine, Cairo University, Egypt. E-Mail: [email protected] Access this Article online Quick Response code Web site: International Journal of Anatomy and Research ISSN 2321-4287 www.ijmhr.org/ijar.htm Received: 28 Sep 2017 Accepted: 20 Oct 2017 Peer Review: 28 Sep 2017 Published (O): 01 Dec 2017 DOI: 10.16965/ijar.2017.465 Revised: None Published (P): 01 Dec 2017 INTRODUCTION allowed movement in three planes. -

Tibial Tuberosity Advancement For
Veterinary Surgery 36:573–586, 2007 Tibial Tuberosity Advancement for Stabilization of the Canine Cranial Cruciate Ligament-Deficient Stifle Joint: Surgical Technique, Early Results, and Complications in 101 Dogs SARAH LAFAVER, DVM,NATHANA.MILLER,DVM, Diplomate ACVS, W. PRESTON STUBBS, DVM, Diplomate ACVS, ROBERT A. TAYLOR, DVM, Diplomate ACVS, and RANDY J. BOUDRIEAU, DVM, Diplomate ACVS & ECVS Objective—To describe the surgical technique, early results and complications of tibial tuberosity advancement (TTA) for treatment for cranial cruciate ligament (CrCL)-deficient stifle joints in dogs. Study Design—Retrospective clinical study. Animals—Dogs (n ¼ 101) with CrCL-deficient stifles (114). Methods—Medical records of 101 dogs that had TTA were reviewed. Complications were recorded and separated into either major or minor complications based on the need for additional surgery. In-hospital re-evaluation of limb function and time to radiographic healing were reviewed. Further follow-up was obtained by telephone interview of owners. Results—Complications occurred in 31.5% of the dogs (12.3% major, 19.3% minor). Major com- plications included subsequent meniscal tear, tibial fracture, implant failure, infection, lick granuloma, incisional trauma, and medial patellar luxation; all major complications were treated with successful outcomes. All but 2 minor complications resolved. The mean time to documented radiographic healing was 11.3 weeks. Final in-hospital re-evaluation of limb function (mean, 13.5 weeks), was recorded for 93 dogs with lameness categorized as none (74.5%), mild (23.5%), mod- erate (2%), and severe (1%). All but 2 owners interviewed were satisfied with outcome and 83.1% reported a marked improvement or a return to pre-injury status. -

Disease of the Bovine Stifle Joint
Disease of the Bovine Stifle Joint A. D. Weaver, B.Sc., Ph.D., Dr.med.vet., F.R.C. VS. Department of Surgery Glasgow University Veterinary Hospital Bearsden, Glasgow, Scotland Introduction Joint Movement Eight out of every ten cattle are lame due to Movement is not simply flexion and extension, foot disease. Upper limb periarticular or articular but also involves some rotation (6,17). While the disease accounts for much of the remainder, in lateral femorotibial surfaces rotate very little, particular stifle and hock joint disease. This paper relative to each other, the medial articular surfaces considers the investigation of stifle lameness and move considerably, so that, when the stifle is discusses some specific conditions. flexed the medial femoral condyle is far forward Anatomy on the medial tibial condyle, over which it has slid to a greater extent than it has rolled. Section of the Several clinical entities occur primarily due to anterior cruciate ligament increases the possible the unusual anatomy of this joint, which must be cranial-caudal movement of the opposing articular outlined first. It is a large joint, divided into surfaces by about 3 cm. Additional section of the femeropatellar and femorotibial compartments, posterior cruciate ligament increases it very slightly which usually communicate with each other via the ( < 1 cm.). Section of the posterior cruciate alone medial femerotibial joint capsule. This communica leads to little or no instability. tion is of obvious importance in septic arthritis Patellar movement is relatively simple. It is at initially of one compartment. There are strong the proximal part of the trochlear ridge when the femorotibial collateral ligaments, and laterally the limb is extended, but, unlike the horse, the medial tendon of the long digital extensor affords patellar ligament does not ride over and catch on additional support. -

Clinical Results of Triple Tibial Osteotomy Treatment in Cranial Cruciate Ligament-Deficient Dogs
Turkish Journal of Veterinary and Animal Sciences Turk J Vet Anim Sci (2020) 44: 495-502 http://journals.tubitak.gov.tr/veterinary/ © TÜBİTAK Research Article doi:10.3906/vet-1912-89 Clinical results of triple tibial osteotomy treatment in cranial cruciate ligament-deficient dogs Mustafa Volkan YAPRAKCI*, Zülfükar Kadir SARITAŞ Department of Surgery, Faculty of Veterinary Medicine, Afyon Kocatepe University, Afyonkarahisar, Turkey Received: 25.12.2019 Accepted/Published Online: 22.02.2020 Final Version: 02.06.2020 Abstract: Canine cranial cruciate ligament ruptures have become common with improved nutrition and nonselective breeding strategies. This study assessed 7 dogs of various breeds and ages with the diagnosis of cranial cruciate ligament ruptures. The triple tibial osteotomy (TTO) method was used to treat the rupture. All of the dogs were admitted to the clinic for varying degrees of lameness. Diagnosis was confirmed using both clinical and radiologic examinations. Body weights of the dogs were between 12 and 65 kg. Informed consent was given by the owners prior to surgery. Postoperatively, early evaluations were made using periodic radiographs and clinical examinations, but long-term evaluations were performed by phone interviews due to economic and other limitations by the owners. The TTO operation method was completed successfully in all of the cases and only with Case 5 was a major complication encountered (tibial tuberosity fracture from the distal pivot hole). In 3 cases, the minor complication of wound contamination occurred due to poor wound care by the owners of the dogs. Owners were advised to restrict mobilization for a week following cast removal. Early, mid-, and long-term postoperative results showed functional recovery in all of the cases. -

Magnetic Resonance Imaging of the Equine Stifle
Magnetic resonance imaging of the equine stifle: normal anatomy Stifle imaging comparison between magnetic resonance images and macroscopic gross-section in a group of nine ponies and nine adult horses: normal anatomy. Drs. Giliam O. van der Straaten1, Dr Simon N. Collins2 , Prof. Dr. Astrid B. Rijkenhuizen1, Dr. Rachel C. Murray2. 1.Department of Equine Sciences, Faculty of Veterinary Medicine, Utrecht University, Yalelaan 12, 3584 CM, Utrecht, The Netherlands. 2. Animal Health Trust, Newmarket, United Kingdom. CB8 7UU UK [email protected] Abstract Introduction: The equine stifle is a complex joint that may be the site of equine lameness, but diagnosis of pain can be frustrating using standard imaging techniques. Magnetic resonance imaging (MRI) of the equine distal limb has led to improvements in understanding of lameness pathogenesis. Until recently, MR imaging has been limited to the distal limb, but recently the use of MRI for clinical evaluation of the equine stifle has been reported. However there is little published information regarding the normal variation in MR anatomy of the equine stifle with which to compare horses with stifle lameness. The aim of this study was to describe normal MRI anatomy and variation in immature and adult equine stifles using comparison between anatomical cross-sectional slices and MR images. Materials and Methods: Post-mortem stifles from 9 immature ponies and 9 adult horses without hind limb lameness underwent MRI using a 1.5 Tesla GE Signa Echospeed MRI system with spoiled gradient echo, T2* gradient echo, short Tau inversion recovery, proton density, and fast dual echo sequences in 3 planes. -

Multiple Ligamentous Injuries of the Canine Stifle Joint: a Literature Review and Clinical Case Study
MULTIPLE LIGAMENTOUS INJURIES OF THE CANINE STIFLE JOINT: A LITERATURE REVIEW AND CLINICAL CASE STUDY by Warrick John Bruce BVSc (Massey) Cert SAO MRCVS A thesis presented to the Faculty of Veterinary Clinical Studies University of Glasgow for the Degree of Master of Veterinary Medicine September 1995 © Warrick John Bruce ProQuest Number: 13834040 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 13834040 Published by ProQuest LLC(2019). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 'fLr-i 10111 S um m ary The aim of this thesis is to detail the clinical, ancillary, surgical and postoperative findings in eleven cases of multiple ligamentous injuries of the canine stifle joint. All cases were presented to the University of Glasgow Veterinary School during the period October 1992 to August 1995. Severe trauma was required to produced multiple ligamentous injury of the stifle joint in nine of the dogs in this series. This resulted from an injury occurring when running at speed, catching the limb in a fence or gate, or from a road traffic accident. -

Ultrasonographic Findings in the Stifle Joint of Active Jumping And
Cur gy: ren lo t o R t e a s e m a u r c e h h Vekens et al., Rheumatology (Sunnyvale) 2016, 6:1 R Rheumatology: Current Research DOI: 10.4172/2161-1149.1000184 ISSN: 2161-1149 Research Article Open Access Ultrasonographic Findings in the Stifle Joint of Active Jumping and Dressage Horses Elke Van der Vekens1*, Erik H J Bergman2, Arie C Hoogendoorn3, Els V Raes1, Bernadette Van Ryssen1, Katrien Vanderperren1and Jimmy H Saunders1 1Department of Medical Imaging, Faculty of Veterinary Medicine, Ghent University, Belgium 2Sporthorse Medical Diagnostic Centre, Hooge Wijststraat 7, 5384 RC Heesch, Netherlands 3De Watermolen, Watermolenweg 5, 7481 VL Haaksbergen, Netherlands *Corresponding author: Elke Van der Vekens, Department of Medical Imaging, Faculty of Veterinary Medicine, Ghent University, Belgium, Tel: 09 264 76 32; E-mail: [email protected] Received date: Oct 21, 2015; Accepted date: Jan 27, 2016; Published date: Jan 29, 2016 Copyright: © 2015 Vekens EV der. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Objective Ultrasonography (US) is frequently used to evaluate the equine stifle joint. Some soft tissue US findings are known to be clinically relevant, while others are considered incidental. These considerations are not always evidence-based. This study aims to describe the US findings observed in the stifle of clinically sound, active jumping and dressage horses. Design: Prospective study Animals: 46 Warmblood horses Procedures: To be included in this study, the horses had to fulfil 4 criteria: (1) in competition at least 1 time/month at national or international level, (2) in full work, (3) free of lameness, (4) no complains of the horse’s performances.