Experiments 32A and 32B COMPTON SCATTERING
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

High-Energy Astrophysics
High-Energy Astrophysics Andrii Neronov October 30, 2017 2 Contents 1 Introduction 5 1.1 Types of astronomical HE sources . .7 1.2 Types of physical processes involved . .8 1.3 Observational tools . .9 1.4 Natural System of Units . 11 1.5 Excercises . 14 2 Radiative Processes 15 2.1 Radiation from a moving charge . 15 2.2 Curvature radiation . 17 2.2.1 Astrophysical example (pulsar magnetosphere) . 19 2.3 Evolution of particle distribution with account of radiative energy loss . 20 2.4 Spectrum of emission from a broad-band distribution of particles . 21 2.5 Cyclotron emission / absorption . 23 2.5.1 Astrophysical example (accreting pulsars) . 23 2.6 Synchrotron emission . 24 2.6.1 Astrophysical example (Crab Nebula) . 26 2.7 Compton scattering . 28 2.7.1 Thomson cross-section . 28 2.7.2 Example: Compton scattering in stars. Optical depth of the medium. Eddington luminosity 30 2.7.3 Angular distribution of scattered waves . 31 2.7.4 Thomson scattering . 32 2.7.5 Example: Compton telescope(s) . 33 2.8 Inverse Compton scattering . 34 2.8.1 Energies of upscattered photons . 34 2.8.2 Energy loss rate of electron . 35 2.8.3 Evolution of particle distribution with account of radiative energy loss . 36 2.8.4 Spectrum of emission from a broad-band distribution of particles . 36 2.8.5 Example: Very-High-Energy gamma-rays from Crab Nebula . 37 2.8.6 Inverse Compton scattering in Klein-Nishina regime . 37 2.9 Bethe-Heitler pair production . 38 2.9.1 Example: pair conversion telescopes. -

Compton Scattering from the Deuteron at Low Energies
SE0200264 LUNFD6-NFFR-1018 Compton Scattering from the Deuteron at Low Energies Magnus Lundin Department of Physics Lund University 2002 W' •sii" Compton spridning från deuteronen vid låga energier (populärvetenskaplig sammanfattning på svenska) Vid Compton spridning sprids fotonen elastiskt, dvs. utan att förlora energi, mot en annan partikel eller kärna. Kärnorna som användes i detta försök består av en proton och en neutron (sk. deuterium, eller tungt väte). Kärnorna bestrålades med fotoner av kända energier och de spridda fo- tonerna detekterades mha. stora Nal-detektorer som var placerade i olika vinklar runt strålmålet. Försöket utfördes under 8 veckor och genom att räkna antalet fotoner som kärnorna bestålades med och antalet spridda fo- toner i de olika detektorerna, kan sannolikheten för att en foton skall spridas bestämmas. Denna sannolikhet jämfördes med en teoretisk modell som beskriver sannolikheten för att en foton skall spridas elastiskt mot en deuterium- kärna. Eftersom protonen och neutronen består av kvarkar, vilka har en elektrisk laddning, kommer dessa att sträckas ut då de utsätts för ett elek- triskt fält (fotonen), dvs. de polariseras. Värdet (sannolikheten) som den teoretiska modellen ger, beror på polariserbarheten hos protonen och neu- tronen i deuterium. Genom att beräkna sannolikheten för fotonspridning för olika värden av polariserbarheterna, kan man se vilket värde som ger bäst överensstämmelse mellan modellen och experimentella data. Det är speciellt neutronens polariserbarhet som är av intresse, och denna kunde bestämmas i detta arbete. Organization Document name LUND UNIVERSITY DOCTORAL DISSERTATION Department of Physics Date of issue 2002.04.29 Division of Nuclear Physics Box 118 Sponsoring organization SE-22100 Lund Sweden Author (s) Magnus Lundin Title and subtitle Compton Scattering from the Deuteron at Low Energies Abstract A series of three Compton scattering experiments on deuterium have been performed at the high-resolution tagged-photon facility MAX-lab located in Lund, Sweden. -

A CMB Polarization Primer
New Astronomy 2 (1997) 323±344 A CMB polarization primer Wayne Hua,1,2 , Martin White b,3 aInstitute for Advanced Study, Princeton, NJ 08540, USA bEnrico Fermi Institute, University of Chicago, Chicago, IL 60637, USA Received 13 June 1997; accepted 30 June 1997 Communicated by David N. Schramm Abstract We present a pedagogical and phenomenological introduction to the study of cosmic microwave background (CMB) polarization to build intuition about the prospects and challenges facing its detection. Thomson scattering of temperature anisotropies on the last scattering surface generates a linear polarization pattern on the sky that can be simply read off from their quadrupole moments. These in turn correspond directly to the fundamental scalar (compressional), vector (vortical), and tensor (gravitational wave) modes of cosmological perturbations. We explain the origin and phenomenology of the geometric distinction between these patterns in terms of the so-called electric and magnetic parity modes, as well as their correlation with the temperature pattern. By its isolation of the last scattering surface and the various perturbation modes, the polarization provides unique information for the phenomenological reconstruction of the cosmological model. Finally we comment on the comparison of theory with experimental data and prospects for the future detection of CMB polarization. 1997 Elsevier Science B.V. PACS: 98.70.Vc; 98.80.Cq; 98.80.Es Keywords: Cosmic microwave background; Cosmology: theory 1. Introduction scale structure we see today. If the temperature anisotropies we observe are indeed the result of Why should we be concerned with the polarization primordial ¯uctuations, their presence at last scatter- of the cosmic microwave background (CMB) ing would polarize the CMB anisotropies them- anisotropies? That the CMB anisotropies are polar- selves. -

7. Gamma and X-Ray Interactions in Matter
Photon interactions in matter Gamma- and X-Ray • Compton effect • Photoelectric effect Interactions in Matter • Pair production • Rayleigh (coherent) scattering Chapter 7 • Photonuclear interactions F.A. Attix, Introduction to Radiological Kinematics Physics and Radiation Dosimetry Interaction cross sections Energy-transfer cross sections Mass attenuation coefficients 1 2 Compton interaction A.H. Compton • Inelastic photon scattering by an electron • Arthur Holly Compton (September 10, 1892 – March 15, 1962) • Main assumption: the electron struck by the • Received Nobel prize in physics 1927 for incoming photon is unbound and stationary his discovery of the Compton effect – The largest contribution from binding is under • Was a key figure in the Manhattan Project, condition of high Z, low energy and creation of first nuclear reactor, which went critical in December 1942 – Under these conditions photoelectric effect is dominant Born and buried in • Consider two aspects: kinematics and cross Wooster, OH http://en.wikipedia.org/wiki/Arthur_Compton sections http://www.findagrave.com/cgi-bin/fg.cgi?page=gr&GRid=22551 3 4 Compton interaction: Kinematics Compton interaction: Kinematics • An earlier theory of -ray scattering by Thomson, based on observations only at low energies, predicted that the scattered photon should always have the same energy as the incident one, regardless of h or • The failure of the Thomson theory to describe high-energy photon scattering necessitated the • Inelastic collision • After the collision the electron departs -
![Arxiv:2008.11688V1 [Astro-Ph.CO] 26 Aug 2020](https://docslib.b-cdn.net/cover/0465/arxiv-2008-11688v1-astro-ph-co-26-aug-2020-410465.webp)
Arxiv:2008.11688V1 [Astro-Ph.CO] 26 Aug 2020
APS/123-QED Cosmology with Rayleigh Scattering of the Cosmic Microwave Background Benjamin Beringue,1 P. Daniel Meerburg,2 Joel Meyers,3 and Nicholas Battaglia4 1DAMTP, Centre for Mathematical Sciences, Wilberforce Road, Cambridge, UK, CB3 0WA 2Van Swinderen Institute for Particle Physics and Gravity, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands 3Department of Physics, Southern Methodist University, 3215 Daniel Ave, Dallas, Texas 75275, USA 4Department of Astronomy, Cornell University, Ithaca, New York, USA (Dated: August 27, 2020) The cosmic microwave background (CMB) has been a treasure trove for cosmology. Over the next decade, current and planned CMB experiments are expected to exhaust nearly all primary CMB information. To further constrain cosmological models, there is a great benefit to measuring signals beyond the primary modes. Rayleigh scattering of the CMB is one source of additional cosmological information. It is caused by the additional scattering of CMB photons by neutral species formed during recombination and exhibits a strong and unique frequency scaling ( ν4). We will show that with sufficient sensitivity across frequency channels, the Rayleigh scattering/ signal should not only be detectable but can significantly improve constraining power for cosmological parameters, with limited or no additional modifications to planned experiments. We will provide heuristic explanations for why certain cosmological parameters benefit from measurement of the Rayleigh scattering signal, and confirm these intuitions using the Fisher formalism. In particular, observation of Rayleigh scattering P allows significant improvements on measurements of Neff and mν . PACS numbers: Valid PACS appear here I. INTRODUCTION direction of propagation of the (primary) CMB photons. There are various distinguishable ways that cosmic In the current era of precision cosmology, the Cosmic structures can alter the properties of CMB photons [10]. -

The Cosmic Microwave Background: the History of Its Experimental Investigation and Its Significance for Cosmology
REVIEW ARTICLE The Cosmic Microwave Background: The history of its experimental investigation and its significance for cosmology Ruth Durrer Universit´ede Gen`eve, D´epartement de Physique Th´eorique,1211 Gen`eve, Switzerland E-mail: [email protected] Abstract. This review describes the discovery of the cosmic microwave background radiation in 1965 and its impact on cosmology in the 50 years that followed. This discovery has established the Big Bang model of the Universe and the analysis of its fluctuations has confirmed the idea of inflation and led to the present era of precision cosmology. I discuss the evolution of cosmological perturbations and their imprint on the CMB as temperature fluctuations and polarization. I also show how a phase of inflationary expansion generates fluctuations in the spacetime curvature and primordial gravitational waves. In addition I present findings of CMB experiments, from the earliest to the most recent ones. The accuracy of these experiments has helped us to estimate the parameters of the cosmological model with unprecedented precision so that in the future we shall be able to test not only cosmological models but General Relativity itself on cosmological scales. Submitted to: Class. Quantum Grav. arXiv:1506.01907v1 [astro-ph.CO] 5 Jun 2015 The Cosmic Microwave Background 2 1. Historical Introduction The discovery of the Cosmic Microwave Background (CMB) by Penzias and Wilson, reported in Refs. [1, 2], has been a 'game changer' in cosmology. Before this discovery, despite the observation of the expansion of the Universe, see [3], the steady state model of cosmology still had a respectable group of followers. -

Thermal History FRW
Cosmology, lect. 7 Thermal History FRW Thermodynamics 43πGpΛ a =−++ρ aa To find solutions a(t) for the 33c2 expansion history of the Universe, for a particular FRW Universe , 8πG kc2 Λ one needs to know how the 22=ρ −+ 2density ρ(t) and pressure p(t) aa 2 aevolve as function of a(t) 33R0 FRW equations are implicitly equivalent to a third Einstein equation, the energy equation, pa ρρ ++30 = ca2 Important observation: the energy equation, pa ρρ ++30 = ca2 is equivalent to stating that the change in internal energy U= ρ cV2 of a specific co-expanding volume V(t) of the Universe, is due to work by pressure: dU= − p dV Friedmann-Robertson-Walker-Lemaitre expansion of the Universe is Adiabatic Expansion Adiabatic Expansion of the Universe: • Implication for Thermal History • Temperature Evolution of cosmic components For a medium with adiabatic index γ: TVγ −1 = cst 4 T Radiation (Photons) γ = T = 0 3 a T 5 = 0 Monatomic Gas γ = T 2 (hydrogen) 3 a Radiation & Matter The Universe is filled with thermal radiation, the photons that were created in The Big Bang and that we now observe as the Cosmic Microwave Background (CMB). The CMB photons represent the most abundant species in the Universe, by far ! The CMB radiation field is PERFECTLY thermalized, with their energy distribution representing the most perfect blackbody spectrum we know in nature. The energy density u(T) is therefore given by the Planck spectral distribution, 81πνh 3 uT()= ν ce3/hν kT −1 At present, the temperature T of the cosmic radiation field is known to impressive precision, -
![Arxiv:2006.06594V1 [Astro-Ph.CO]](https://docslib.b-cdn.net/cover/5120/arxiv-2006-06594v1-astro-ph-co-585120.webp)
Arxiv:2006.06594V1 [Astro-Ph.CO]
Mitigating the optical depth degeneracy using the kinematic Sunyaev-Zel'dovich effect with CMB-S4 1, 2, 2, 1, 3, 4, 5, 6, 5, 6, Marcelo A. Alvarez, ∗ Simone Ferraro, y J. Colin Hill, z Ren´eeHloˇzek, x and Margaret Ikape { 1Berkeley Center for Cosmological Physics, Department of Physics, University of California, Berkeley, CA 94720, USA 2Lawrence Berkeley National Laboratory, One Cyclotron Road, Berkeley, CA 94720, USA 3Department of Physics, Columbia University, New York, NY, USA 10027 4Center for Computational Astrophysics, Flatiron Institute, New York, NY, USA 10010 5Dunlap Institute for Astronomy and Astrophysics, University of Toronto, 50 St George Street, Toronto ON, M5S 3H4, Canada 6David A. Dunlap Department of Astronomy and Astrophysics, University of Toronto, 50 St George Street, Toronto ON, M5S 3H4, Canada The epoch of reionization is one of the major phase transitions in the history of the universe, and is a focus of ongoing and upcoming cosmic microwave background (CMB) experiments with im- proved sensitivity to small-scale fluctuations. Reionization also represents a significant contaminant to CMB-derived cosmological parameter constraints, due to the degeneracy between the Thomson- scattering optical depth, τ, and the amplitude of scalar perturbations, As. This degeneracy subse- quently hinders the ability of large-scale structure data to constrain the sum of the neutrino masses, a major target for cosmology in the 2020s. In this work, we explore the kinematic Sunyaev-Zel'dovich (kSZ) effect as a probe of reionization, and show that it can be used to mitigate the optical depth degeneracy with high-sensitivity, high-resolution data from the upcoming CMB-S4 experiment. -
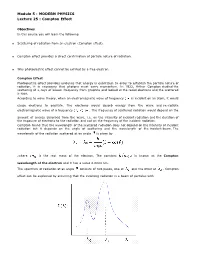
Compton Effect
Module 5 : MODERN PHYSICS Lecture 25 : Compton Effect Objectives In this course you will learn the following Scattering of radiation from an electron (Compton effect). Compton effect provides a direct confirmation of particle nature of radiation. Why photoelectric effect cannot be exhited by a free electron. Compton Effect Photoelectric effect provides evidence that energy is quantized. In order to establish the particle nature of radiation, it is necessary that photons must carry momentum. In 1922, Arthur Compton studied the scattering of x-rays of known frequency from graphite and looked at the recoil electrons and the scattered x-rays. According to wave theory, when an electromagnetic wave of frequency is incident on an atom, it would cause electrons to oscillate. The electrons would absorb energy from the wave and re-radiate electromagnetic wave of a frequency . The frequency of scattered radiation would depend on the amount of energy absorbed from the wave, i.e. on the intensity of incident radiation and the duration of the exposure of electrons to the radiation and not on the frequency of the incident radiation. Compton found that the wavelength of the scattered radiation does not depend on the intensity of incident radiation but it depends on the angle of scattering and the wavelength of the incident beam. The wavelength of the radiation scattered at an angle is given by .where is the rest mass of the electron. The constant is known as the Compton wavelength of the electron and it has a value 0.0024 nm. The spectrum of radiation at an angle consists of two peaks, one at and the other at . -

Compton Scattering from Low to High Energies
Compton Scattering from Low to High Energies Marc Vanderhaeghen College of William & Mary / JLab HUGS 2004 @ JLab, June 1-18 2004 Outline Lecture 1 : Real Compton scattering on the nucleon and sum rules Lecture 2 : Forward virtual Compton scattering & nucleon structure functions Lecture 3 : Deeply virtual Compton scattering & generalized parton distributions Lecture 4 : Two-photon exchange physics in elastic electron-nucleon scattering …if you want to read more details in preparing these lectures, I have primarily used some review papers : Lecture 1, 2 : Drechsel, Pasquini, Vdh : Physics Reports 378 (2003) 99 - 205 Lecture 3 : Guichon, Vdh : Prog. Part. Nucl. Phys. 41 (1998) 125 – 190 Goeke, Polyakov, Vdh : Prog. Part. Nucl. Phys. 47 (2001) 401 - 515 Lecture 4 : research papers , field in rapid development since 2002 1st lecture : Real Compton scattering on the nucleon & sum rules IntroductionIntroduction :: thethe realreal ComptonCompton scatteringscattering (RCS)(RCS) processprocess ε, ε’ : photon polarization vectors σ, σ’ : nucleon spin projections Kinematics in LAB system : shift in wavelength of scattered photon Compton (1923) ComptonCompton scatteringscattering onon pointpoint particlesparticles Compton scattering on spin 1/2 point particle (Dirac) e.g. e- Klein-Nishina (1929) : Thomson term ΘL = 0 Compton scattering on spin 1/2 particle with anomalous magnetic moment Stern (1933) Powell (1949) LowLow energyenergy expansionexpansion ofof RCSRCS processprocess Spin-independent RCS amplitude note : transverse photons : Low -

Science from Litebird
Prog. Theor. Exp. Phys. 2012, 00000 (27 pages) DOI: 10.1093=ptep/0000000000 Science from LiteBIRD LiteBIRD Collaboration: (Fran¸coisBoulanger, Martin Bucher, Erminia Calabrese, Josquin Errard, Fabio Finelli, Masashi Hazumi, Sophie Henrot-Versille, Eiichiro Komatsu, Paolo Natoli, Daniela Paoletti, Mathieu Remazeilles, Matthieu Tristram, Patricio Vielva, Nicola Vittorio) 8/11/2018 ............................................................................... The LiteBIRD satellite will map the temperature and polarization anisotropies over the entire sky in 15 microwave frequency bands. These maps will be used to obtain a clean measurement of the primordial temperature and polarization anisotropy of the cosmic microwave background in the multipole range 2 ` 200: The LiteBIRD sensitivity will be better than that of the ESA Planck satellite≤ by≤ at least an order of magnitude. This document summarizes some of the most exciting new science results anticipated from LiteBIRD. Under the conservatively defined \full success" criterion, LiteBIRD will 3 measure the tensor-to-scalar ratio parameter r with σ(r = 0) < 10− ; enabling either a discovery of primordial gravitational waves from inflation, or possibly an upper bound on r; which would rule out broad classes of inflationary models. Upon discovery, LiteBIRD will distinguish between two competing origins of the gravitational waves; namely, the quantum vacuum fluctuation in spacetime and additional matter fields during inflation. We also describe how, under the \extra success" criterion using data external to Lite- BIRD, an even better measure of r will be obtained, most notably through \delensing" and improved subtraction of polarized synchrotron emission using data at lower frequen- cies. LiteBIRD will also enable breakthrough discoveries in other areas of astrophysics. We highlight the importance of measuring the reionization optical depth τ at the cosmic variance limit, because this parameter must be fixed in order to allow other probes to measure absolute neutrino masses. -

The Basic Interactions Between Photons and Charged Particles With
Outline Chapter 6 The Basic Interactions between • Photon interactions Photons and Charged Particles – Photoelectric effect – Compton scattering with Matter – Pair productions Radiation Dosimetry I – Coherent scattering • Charged particle interactions – Stopping power and range Text: H.E Johns and J.R. Cunningham, The – Bremsstrahlung interaction th physics of radiology, 4 ed. – Bragg peak http://www.utoledo.edu/med/depts/radther Photon interactions Photoelectric effect • Collision between a photon and an • With energy deposition atom results in ejection of a bound – Photoelectric effect electron – Compton scattering • The photon disappears and is replaced by an electron ejected from the atom • No energy deposition in classical Thomson treatment with kinetic energy KE = hν − Eb – Pair production (above the threshold of 1.02 MeV) • Highest probability if the photon – Photo-nuclear interactions for higher energies energy is just above the binding energy (above 10 MeV) of the electron (absorption edge) • Additional energy may be deposited • Without energy deposition locally by Auger electrons and/or – Coherent scattering Photoelectric mass attenuation coefficients fluorescence photons of lead and soft tissue as a function of photon energy. K and L-absorption edges are shown for lead Thomson scattering Photoelectric effect (classical treatment) • Electron tends to be ejected • Elastic scattering of photon (EM wave) on free electron o at 90 for low energy • Electron is accelerated by EM wave and radiates a wave photons, and approaching • No