Download File
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

To Download As
Registered with the Reg. No. TN/CH(C)/374/18-20 Registrar of Newspapers Licenced to post without prepayment for India under R.N.I. 53640/91 Licence No. TN/PMG(CCR)/WPP-506/18-20 Publication: 1st & 16th of every month Rs. 5 per copy (Annual Subscription: Rs. 100/-) INSIDE Short ‘N’ Snappy The Music Season The Bharati Debate Cre-A Ramakrishnan Ranji Trophy, 1934 www.madrasmusings.com WE CARE FOR MADRAS THAT IS CHENNAI Vol. XXX No. 12 December 1-15, 2020 A new reservoir OLD AND NEW ¶ after 76 years n November 21, 2020, the Poondi Reservoir scheme was as much and yet it took us 76 OUnion Home Minister, approved in August 1940 and years to build a new facility. Amit Shah, inaugurated the the foundation stone laid on It is not as though nothing fifth reservoir of the city, locat- the 8th of that month. The has been done in the interim. ed at Thervoy Kandigai in Thi- construction was completed We have had the Telugu Ganga ruvallur District. It will have four years later, by when Sa- scheme, we have harnessed a capacity of one thousand tyamurti was dead. The storage the Palar, requisitioned the million cubic feet (1 tmcft) and facility was rather appropriately Veeranam lake and also got is expected to go a long way in named Satyamurti Sagar in his the Chemparampakkam water- solving the water crises that memory. With a capacity of body to cater to our insatiable the city faces in most years. It 2,573 mcft, it is of course small- thirst. -

Physical and Nutritional Evaluation of Freeze Dried Jamun Pulp Powder Stored at Different Package and Storage Conditions
Indian Journal of Natural Sciences www.tnsroindia.org. ©IJONS Vol.7 / Issue 39 / December 2016 International Bimonthly ISSN: 0976 – 0997 RESEARCH ARTICLE Physical and Nutritional Evaluation of Freeze Dried Jamun Pulp Powder Stored at Different Package and Storage Conditions S.Reginold Jebitta1*, M.Ramanathan2 and P.Rajkumar2 1Department of Food Processing and Engineering, Karunya University, Coimbatore,TamilNadu,India. 2Tamil Nadu Agricultural University, Coimbatore, TamilNadu,India. Received: 21 Aug 2016 Revised: 9 Sep 2016 Accepted: 20 Oct 2016 Address for correspondence S.Reginold Jebitta Department of Food Processing and Engineering, Karunya University,Coimbatore, TamilNadu,India. Email:[email protected] This is an Open Access Journal / article distributed under the terms of the Creative Commons Attribution License (CC BY-NC-ND 3.0) which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. All rights reserved. ABSTRACT Jamun Syzygium cumini is a seasonable fruit which is widely distributed in forest tree in India and other tropical region. It is consumed as fresh for its nutritive value. The fruit is rich in anthocyanin, tannins, Gallic acid and many Phytonutrients. High fluctuation in market price on season times is mainly due to wastage. Drying is done to enhance the storage stability, reduce transport weight and retains its nutritive values. Freeze drying was conducted at -40°C the dried powder was packed at two different packaging material Metalized Polyester Pouches (MPE) and Low Density polyethylene Pouches (LDPE). Under three various storage conditions were S1 (Ambient temperature), S2 (ambient temperature in Dark), S3 (low temperature 4°C). On comparing the results of storage study it was found that Moisture content 3.15 %, Bulk density 0.60, solubility 69.85 and flow ability 31.47% was found at the end of six month in S3 condition packed in MPE. -
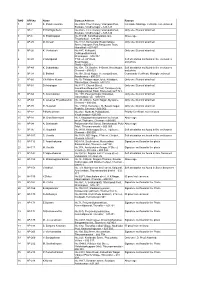
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -

Irrigation Projects of Tamil Nadu from 2001-2021
IRRIGATION PROJECTS OF TAMIL NADU FROM 2001-2021 NAME – VRINDA GUPTA INSTITUTION – K.R. MANGALAM UNIVERSITY 1 ABSTRACT From the ancient times water is always most important for agriculture purpose for growing crops. Since thousand years, humans have relied on agriculture to feed their communities and they have needed irrigation to water their crops. Irrigation includes artificially applying water to the land to enhance the growing of crops. Over the years, irrigation has come in many different forms in countries all over the world. Irrigation projects involves hydraulic structures which collect, convey and deliver water to those areas on which crops are grown. Irrigation projects unit may starts from a small farm unit to those serving extensive areas of millions of hectares. Irrigation projects consist of two types first a small irrigation project and second a large irrigation project. Small irrigation project includes a low diversion or an inexpensive pumping plant along with small channels and some minor control structures. Large irrigation project includes a huge dam, a large storage reservoir, hundreds kilometers of canals, branches and distributaries, control structures and other works. In this paper we discussing about irrigation plan of Tamil Nadu from 2001-2021. INTRODUCTION Water is the important or elixir of life, a precious gift of nature to humans and millions of other species living on the earth. It is hard to find in most part of the world. 4% of India’s land area in Tamil Nadu and inhabited by 6% of India’s population but water resources in India is only 2.5%. In Tamil Nadu, water is a serious limiting factor for agriculture growth which leads to irrigation reduces risk in farming, increases crop productivity, provides higher employment opportunities to the rural areas and increases farmer income. -

Pre Matric Scholarship 2019-2020 - Fresh Sl
Pre Matric Scholarship 2019-2020 - Fresh Sl. Name / Father Applicant Id Institute name Address Disb.Amt no Name BABA VIDALAYA N& P ( CUDDALORE - 48 Nellukadai street Parangipettai 1 TN201920001530939 HUMAIRA /SHAALISHAHIB TAMIL NADU ) / 33181000605 Bhuvanagiri Taluk 1000 BABA VIDALAYA N& P ( CUDDALORE - 2 TN201920002536672 FATHIMA /NAZRHUSSAIN TAMIL NADU ) / 33181000605 38, Kaziyar Street, Parangipettai. 1000 MOHAMED HAFEEL BABA VIDALAYA N& P ( CUDDALORE - 8/1, JAIYEIBAVA STREET, 3 TN201920006709903 /MOHAMED BASHA TAMIL NADU ) / 33181000605 PARANGIPETTAI. 1000 BABA VIDALAYA N& P ( CUDDALORE - 4 TN201920001097372 ADHNAN /Syed Musthafa TAMIL NADU ) / 33181000605 34 Vathiyapalli Street-Parangipettai 1000 MOHAMEDHASHIM BABA VIDALAYA N& P ( CUDDALORE - 5 TN201920003629460 /Abdul Haleem TAMIL NADU ) / 33181000605 33-2 nd Street PK Road -Karaikal 1000 MOHAMED HAMADHA BABA VIDALAYA N& P ( CUDDALORE - 20/47, KUTTAIYA CHETTY ST., 6 TN201920006429121 /MOHAMED BASHA TAMIL NADU ) / 33181000605 PARANGIPETTAI. 1000 BABA VIDALAYA N& P ( CUDDALORE - 7 TN201920002592363 MANHA /Noor Sukkur Ali TAMIL NADU ) / 33181000605 No 21,Kajiyar Street-Parangipettai 1000 BABA VIDALAYA N& P ( CUDDALORE - 8 TN201920001097647 ARFAN /Syed Musrhafa TAMIL NADU ) / 33181000605 34 Vathiyapalli Dtreet Parangipettai 1000 MOHAMED ASEED BABA VIDALAYA N& P ( CUDDALORE - 9 TN201920001348752 /YOSUF ALI TAMIL NADU ) / 33181000605 11/3,Kollankadai St., Parangipettai. 1000 MOHAMED HISHAAM A H BABA VIDALAYA N& P ( CUDDALORE - 10 TN201920003628594 /Abdul Haleem TAMIL NADU ) / 33181000605 -

Status of Wetlands and Wetland Birds in Selected Districts of Tamilnadu
STATUS OF WETLANDS AND WETLAND BIRDS IN SELECTED DISTRICTS OF TAMILNADU SÁLIM ALI CENTRE FOR ORNITHOLOGY & NATURAL ISTORY STATUS OF WETLANDS AND WETLAND BIRDS IN SELECTED DISTRICTS OF TAMILNADU Investigators LALITHA VIJAYAN & S. N. PRASAD Research students N. SRIDHARAN & M. BUBESH GUPTHA SÁLIM ALI CENTRE FOR ORNITHOLOGY & NATURAL HISTORY 2006 1 CONTENTS 1. Introduction-------------------------------------------------------------------------- -4 Objectives -----------------------------------------------------------------------------9 2. Study area -----------------------------------------------------------------------------9 Tamil Nadu Intensive study area 3. Some of the common wetland birds in the study area --------------------------14 4. Methodology -------------------------------------------------------------------------18 Data Analysis - Diversity - Commonness - Relative Dominance 5. Literature Review---------------------------------------------------------------------19 6. Results----------------------------------------------------------------------------------21 Distribution of wetland birds - District-wise - Migratory birds - Common birds - Species diversity - Threatened birds - Vegetation 7. Discussion------------------------------------------------------------------------------57 8. Threats to wetlands--------------------------------------------------------------------60 9. Conservation ---------------------------------------------------------------------------60 10. Reference -----------------------------------------------------------------------------62 -

Cuddalore District
DISTRICT DIAGNOSTIC REPORT (DDR) Tamil Nadu Rural Transformation Project Cuddalore District 1 1 DDR - CUDDALORE 2 DDR - CUDDALORE Table of Contents S.No Contents Page No 1.0 Introduction 10 1.1 About Tamil Nadu Rural Transformation Project - TNRTP 1.2 About District Diagnostic Study – DDS 2.0 CUDDALORE DISTRICT 12 2.1 District Profile 3.0 Socio Demographic profile 14 3.1 Population 3.2 Sex Ratio 3.3 Literacy rate 3.4 Occupation 3.5 Community based institutions 3.6 Farmer Producer Organisations (FPOs) 4.0 District economic profile 21 4.1 Labour and Employment 4.2 Connectivity 5.0 GEOGRAPHIC PROFILE 25 5.1 Topography 5.2 Land Use Pattern of the District 5.3 Land types 5.4 Climate and Rainfall 5.5 Disaster Vulnerability 5.6 Soil 5.7 Water Resources 31 DDR - CUDDALORE S.No Contents Page No 6.0 STATUS OF GROUND WATER 32 7.0 FARM SECTOR 33 7.1 Land holding pattern 7.2 Irrigation 7.3 Cropping pattern and Major crops 7.4 Block wise (TNRTP) cropping area distribution 7.5 Prioritization of crops 7.6 Crop wise discussion 8.0 MARKETING AND STORAGE INFRASTRUCTURE 44 9.0 AGRIBUSINESS OPPORTUNITIES 46 10.0 NATIONAL AND STATE SCHEMES ON AGRICULTURE 48 11.0 RESOURCE INSTITUTIONS 49 12.0 ALLIED SECTORS 50 12.1 Animal Husbandry and Dairy development 12.2 Poultry 12.3 Fisheries 12.4 Sericulture 4 DDR - CUDDALORE S.No Contents Page No 13.0 NON-FARM SECTORS 55 13.1 Industrial scenario in the district 13.2 MSME clusters 13.3 Manufacturing 13.4 Service sectors 13.5 Tourism 14.0 SKILL GAPS 65 15.0 BANKING AND CREDIT 67 16.0 COMMODITY PRIORITISATION 69 SWOT ANALYSIS 72 CONCLUSION 73 ANNEXURE 76 51 DDR - CUDDALORE List of Tables Table Number and details Page No Table .1. -

Annexure – 1 List of Tourist Places in Tamil Nadu -..::Tamilnadu Tourism
Annexure – 1 List of Tourist Places in Tamil Nadu Name of Beaches Eco- Tourism Wildlife / Bird Others Art & Culture / Heritage Pilgrim Centers Hills the District (1) (2) Sanctuary (4 & 5) (6) Stations ( 3) Chennai 1.Elliots Beach 1.Guindy, 1.High Court of 1.St. George Fort 1. AshtalakshmiTemple, 2. Marina Beach Children’s Park Madras 2. Ameer Mahal Chennai2.KapaleeswararTemple, 3. Light House 2.SnakePark 2.Madras University 3. VivekanandarIllam Mylapore 3.Parthasarathi Temple, 3.Rippon Building 4.Valluvar Kottam Triplicane 4. TidelPark 5.Gandhi Mandapam 4.Vadapalani Murugan Temple 5.BirlaKolarangam 6.Kamarajar Memorial 5.St.Andru’s Church 6.Lait Kala Academy 7.M.G.R Memorial 6.Santhome Catherdral 7. AnnanagarTower 8.Periyar Memorial 7.Makka Mosque, Thousand Lights 8.Apollo Hospital 9.Connemara public library 8.Shirdi SaibabaTemple, Mylapore 9.SankaraNethralaya 10.Govt. Museum, Egmore 9.KalingambalTemple, Parry’s 10. Adayar cancer 11.Fort Museum 10.Marundeeswarar Temple, Hospital and 12. Kalashethra Tiruvanmiyur Institute 13. Rail Museum, Perambur 11.Jain Temple 11. Vijaya Hospital, 14. Rajaji Hall 12.Iyyappan Vadaplani 15.Anna Square Temple,Mahalingapuram&Annanagar 12.Sankara 16.Barathiyar Memorial 13.Thirumalai TirupattyDevasthanam, NethralayaEye 17. M.G.R. Illam T. Nagar Hospital. 18. Govt. Fine Arts Collage. 14.Buddhavihar, Egmore 13. Adyar 15.Madhiya Kailash Temple, Adyar BaniyanTree 16.RamakrishnaTemple 14. Arvind Eye 17. Velankanni Church, Beasant Nagar Hospital 18.St. George Catherdral 19. BigMosque,Triplicane. Name of Beaches Eco- Tourism Wildlife / Bird Others Art & Culture / Heritage Pilgrim Centers Hills the District Sanctuary Stations Ariyalur 1.Karaivetti 1.Fossile Museum 1.JayankondamPalace 1.Adaikala Madha Shrine, Elakurichi Bird Sanctuary 2. -

04092018B95x76lefinaladditio
No.IA-J-11011/207/2018-IA-II(I) Goverment of India Minister of Enviroment,Forest and Climate Change Impact Assessment Division *** Indira Paryavaran Bhavan, Vayu Wing,3rd Floor,Aliganj, Jor Bagh Road,New Delhi-110003 27 Jul 2018 To, M/s CRIMSUN ORGANICS PRIVATE LIMITED Plot No. C-10 and C-11, SIPCOT industrial complex, Kudikadu village, Cuddalore Taluk, Cuddalore District. Tamil nadu, Cuddalore-607005 Tamil Nadu Tel.No.04142-239933; Email:[email protected] Sir/Madam, This has reference to the proposal submitted in the Ministry of Environment, Forest and Climate Change to prescribe the Terms of Reference (TOR) for undertaking detailed EIA study for the purpose of obtaining Environmental Clearance in accordance with the provisions of the EIA Notification, 2006. For this purpose, the proponent had submitted online information in the prescribed format (Form-1 ) along with a Pre-feasibility Report. The details of the proposal are given below: 1. Proposal No.: IA/TN/IND2/75539/2018 Proposed Manufacturing of Specialty Chemicals 2. Name of the Proposal: and Agro Chemical Products 3. Category of the Proposal: Industrial Projects - 2 4. Project/Activity applied for: 5(b) Pesticides industry and pesticide specific intermediates (excluding formulations) 5. Date of submission for TOR: 23 Jun 2018 In this regard, under the provisions of the EIA Notification 2006 as amended, the Standard TOR for the purpose of preparing environment impact assessment report and environment management plan for obtaining prior environment clearance is prescribed with public consultation as follows: STANDARD TERMS OF REFERENCE (TOR) FOR EIA/EMP REPORT FOR PROJECTS/ ACTIVITIES REQUIRING ENVIRONMENT CLEARANCE 5(b): STANDARD TERMS OF REFERENCE FOR CONDUCTING ENVIRONMENT IMPACT ASSESSMENT STUDY FOR PESTICIDES INDUSTRY AND PESTICIDE SPECIFIC INTERMEDIATES (EXCLUDING FORMULATIONS)AND INFORMATION TO BE INCLUDED IN EIA/EMP REPORT A. -

Pios and Appellate Authorities for O/O the District Collector, Cuddalore
List of Public Information Officer’s / Assistant Public Information Officers/ Appellate Authority appointed in various agencies / Departments in Cuddalore District . Revenue Department : AREAS OF OFFICER APPOINTED OFFICER APPOINTED RESPONSIBILITY AS PUBLIC AS APPEALLATE INFORMATION AUTHORITY OFFICER 1)All subjects dealt in Personal Assistant District Revenue Collectorate (General) to the Collector, Officer Cuddalore. Cuddalore 2) All matters relating to District Supply Officer, Civil Supplies and Cuddalore -do- Public Distribution System. 3) All matters relating to District Adi-Diravidar Adi-Dravidar and Tribal Welfare Officer, -do- Welfare Cuddalore 4) All matters relating to District Backward classes -do- Backward Classes and and Minorities Welfare Minorities Welfare Officer 5) All matters relating to Assistant Commissioner -do- Prohibition & Excise (Excise) Cuddalore 6) All matters relating to Special Deputy Collector Public Grievances and (Social Security Scheme), -do- Social Security Scheme. Cuddalore 7) All matters relating to Personal Assistant Accounts of Revenue (Accounts) to the -do- Department. Collector, Cuddalore 8) All matters relating to Inspection Cell Officer, the functioning of the Cuddalore -do- Inspection Cell. 9) All matters relating to Special Deputy Collector Stamps & Deficit Stamp (Stamps), Cuddalore Duty under Stamp Act. -do- 1) With respect to all Personal Assistant to Sub-Collector, subjects handled and Sub-Collector, Chidambaram functions performed by the Chidambaram. Sub-Collector, Chidambaram. With respect -

Download PDF from Description Box
Today’s TNA ➢ Download PDF from Description Box 1. MCQs Revision 2. BANKING & FINANCE 3. Appointments 4. State in News 5. National & International News 6. Science & Tech 7. Static GK: Tamil Nadu 8. Projection of GDP Daily Current Affairs @7:30AM Live Q.1 Article ________ recognises Hindi as the official language of India. ी को माꅍयता देता ै। ﴂQ.1 अनु楍छेद ________ भारत की आधिकाररक भाषा के 셂प मᴂ ह द a) 110 b) 112 c) 352 d) 343 e) 370 ➢ Hindi Divas is celebrated on 14 September because on this day in 1949, the Constituent Assembly of India had adopted Hindi written in Devanagari script as the official language of the Republic of India. Daily Current Affairs @7:30AM Live Q.2 Streets for People Challenge was launched by which of the following ministries ? a) Ministry of Environment, Forest and Climate Change b) Ministry of Home Affairs c) Ministry of Rural Development d) Ministry of Housing and Urban Affairs e) Ministry of Road Transport and Highways ➢ The Streets for People Challenge is the response to the need for making the cities more walkable and pedestrian-friendly. Daily Current Affairs @7:30AM Live Q.3 Which state government has launched My Family, My Responsibility’ campaign to tackle coronavirus. a) Madhya Pradesh b) Maharashtra c) Delhi d) Kerala e) Karnataka ➢ BMC, the authorities will reach out to every family door-to-door to check temperature and oxygen levels. Daily Current Affairs @7:30AM Live State in News ➢ Maharashtra govt accords ambulance status to vehicles carrying medical oxygen for one year ▪ For an uninterrupted supply of oxygen, vehicles carrying oxygen cylinders for medical use will be treated at par with ambulances as emergency vehicles in Maharashtra for a period of 1 year. -

General-STATIC-BOLT.Pdf
oliveboard Static General Static Facts CLICK HERE TO PREPARE FOR IBPS, SSC, SBI, RAILWAYS & RBI EXAMS IN ONE PLACE Bolt is a series of GK Summary ebooks by Oliveboard for quick revision oliveboard.in www.oliveboard.in Table of Contents International Organizations and their Headquarters ................................................................................................. 3 Organizations and Reports .......................................................................................................................................... 5 Heritage Sites in India .................................................................................................................................................. 7 Important Dams in India ............................................................................................................................................... 8 Rivers and Cities On their Banks In India .................................................................................................................. 10 Important Awards and their Fields ............................................................................................................................ 12 List of Important Ports in India .................................................................................................................................. 12 List of Important Airports in India ............................................................................................................................. 13 List of Important