Glyceroglycolipids from Euphorbia Nicaeensis All. with Antiinflamatory Activity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cally Plant List a ACIPHYLLA Horrida
Cally Plant List A ACIPHYLLA horrida ACONITUM albo-violaceum albiflorum ABELIOPHYLLUM distichum ACONITUM cultivar ABUTILON vitifolium ‘Album’ ACONITUM pubiceps ‘Blue Form’ ACAENA magellanica ACONITUM pubiceps ‘White Form’ ACAENA species ACONITUM ‘Spark’s Variety’ ACAENA microphylla ‘Kupferteppich’ ACONITUM cammarum ‘Bicolor’ ACANTHUS mollis Latifolius ACONITUM cammarum ‘Franz Marc’ ACANTHUS spinosus Spinosissimus ACONITUM lycoctonum vulparia ACANTHUS ‘Summer Beauty’ ACONITUM variegatum ACANTHUS dioscoridis perringii ACONITUM alboviolaceum ACANTHUS dioscoridis ACONITUM lycoctonum neapolitanum ACANTHUS spinosus ACONITUM paniculatum ACANTHUS hungaricus ACONITUM species ex. China (Ron 291) ACANTHUS mollis ‘Long Spike’ ACONITUM japonicum ACANTHUS mollis free-flowering ACONITUM species Ex. Japan ACANTHUS mollis ‘Turkish Form’ ACONITUM episcopale ACANTHUS mollis ‘Hollard’s Gold’ ACONITUM ex. Russia ACANTHUS syriacus ACONITUM carmichaelii ‘Spätlese’ ACER japonicum ‘Aconitifolium’ ACONITUM yezoense ACER palmatum ‘Filigree’ ACONITUM carmichaelii ‘Barker’s Variety’ ACHILLEA grandifolia ACONITUM ‘Newry Blue’ ACHILLEA ptarmica ‘Perry’s White’ ACONITUM napellus ‘Bergfürst’ ACHILLEA clypeolata ACONITUM unciniatum ACIPHYLLA monroi ACONITUM napellus ‘Blue Valley’ ACIPHYLLA squarrosa ACONITUM lycoctonum ‘Russian Yellow’ ACIPHYLLA subflabellata ACONITUM japonicum subcuneatum ACONITUM meta-japonicum ADENOPHORA aurita ACONITUM napellus ‘Carneum’ ADIANTUM aleuticum ‘Japonicum’ ACONITUM arcuatum B&SWJ 774 ADIANTUM aleuticum ‘Miss Sharples’ ACORUS calamus ‘Argenteostriatus’ -

Euphorbia Subg
ФЕДЕРАЛЬНОЕ ГОСУДАРСТВЕННОЕ БЮДЖЕТНОЕ УЧРЕЖДЕНИЕ НАУКИ БОТАНИЧЕСКИЙ ИНСТИТУТ ИМ. В.Л. КОМАРОВА РОССИЙСКОЙ АКАДЕМИИ НАУК На правах рукописи Гельтман Дмитрий Викторович ПОДРОД ESULA РОДА EUPHORBIA (EUPHORBIACEAE): СИСТЕМА, ФИЛОГЕНИЯ, ГЕОГРАФИЧЕСКИЙ АНАЛИЗ 03.02.01 — ботаника ДИССЕРТАЦИЯ на соискание ученой степени доктора биологических наук САНКТ-ПЕТЕРБУРГ 2015 2 Оглавление Введение ......................................................................................................................................... 3 Глава 1. Род Euphorbia и основные проблемы его систематики ......................................... 9 1.1. Общая характеристика и систематическое положение .......................................... 9 1.2. Краткая история таксономического изучения и формирования системы рода ... 10 1.3. Основные проблемы систематики рода Euphorbia и его подрода Esula на рубеже XX–XXI вв. и пути их решения ..................................................................................... 15 Глава 2. Материал и методы исследования ........................................................................... 17 Глава 3. Построение системы подрода Esula рода Euphorbia на основе молекулярно- филогенетического подхода ...................................................................................................... 24 3.1. Краткая история молекулярно-филогенетического изучения рода Euphorbia и его подрода Esula ......................................................................................................... 24 3.2. Результаты молекулярно-филогенетического -

Downloaded from Brill.Com10/07/2021 07:28:06AM Via Free Access - 152 P
Bijdragen tot de Dierkunde, 64 (3) 151-162 (1994) SPB Academie Publishing bv, The Hague A standardized description of European Sminthuridae (Collembola, Symphypleona), 2: review of four species of the genera Allacma and Spatulosminthurus Pierre Nayrolles Laboratoire de Zoologie, Ecobiologie des Arthropodes édaphiques, UPR CNRS 90 14, Université Paul Sabatier, 118 route de Narbonne, F-31062 Toulouse Cédex, France Keywords: Collembola, Symphypleona, Allacma, Spatulosminthurus, taxonomy, chaetotaxy, Europe Abstract et al., 1970), thick cuticle with a particular archi- tecture, and well-developed tracheal system with According to our standard of the appendicular chaetotaxy, the chiasmata at the forelegs. Moreover, Betsch & following species are redescribed: Allacma fusca (Linné, 1758), Waller (in press) point out the presence of a secon- Allacma gallica (Carl, 1899), Spatulosminthurus lesnei (Carl, dary (ontogenetic) neochaetosis on the great abdo- 1899), and Spatulosminthurus betschi Nayrolles, 1990. men as a synapomorphy of these three genera (like- ly the consequence of an ontogenetic delay in- The Résumé volving originally primary setae). following putative evolved characters can be added: setae (AD)iO, (AD)i+ 1, and (AD)i + 2 small and slender Nous redécrivons, d’après la standardisation donnée pour la chétotaxie appendiculaire, les espèces suivantes: Allacma fusca on a large base, and mucronal anterior lamella (Linné, 1758), Allacma gallica (Cari, 1899), Spatulosminthurus double. lesnei (Cari, 1899) et Spatulosminthurus betschi Nayrolles, I recall the abbreviations which correspond 1990. neither to setal symbols nor to the legend of the chaetotaxic tables; these were accurately explained in my first Introduction paper. abd. = abdomen This is the second part of a series dealing with the ad. -

Sex Expression in a Rainforest Understory Herb, Begonia Urophylla John Cozza University of Miami, [email protected]
University of Miami Scholarly Repository Open Access Dissertations Electronic Theses and Dissertations 2008-12-18 Sex Expression in a Rainforest Understory Herb, Begonia urophylla John Cozza University of Miami, [email protected] Follow this and additional works at: https://scholarlyrepository.miami.edu/oa_dissertations Recommended Citation Cozza, John, "Sex Expression in a Rainforest Understory Herb, Begonia urophylla" (2008). Open Access Dissertations. 186. https://scholarlyrepository.miami.edu/oa_dissertations/186 This Open access is brought to you for free and open access by the Electronic Theses and Dissertations at Scholarly Repository. It has been accepted for inclusion in Open Access Dissertations by an authorized administrator of Scholarly Repository. For more information, please contact [email protected]. UNIVERSITY OF MIAMI SEX EXPRESSION IN A RAINFOREST UNDERSTORY HERB, BEGONIA UROPHYLLA By John Cozza A DISSERTATION Submitted to the Faculty of the University of Miami in partial fulfillment of the requirements for the degree of Doctor of Philosophy Coral Gables, Florida December 2008 ©2008 John Cozza All Rights Reserved UNIVERSITY OF MIAMI A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy SEX EXPRESSION IN A RAINFOREST UNDERSTORY HERB, BEGONIA UROPHYLLA John Cozza Approved: ________________ _________________ Carol C. Horvitz, Ph.D. Terri A. Scandura, Ph.D. Professor of Biology Dean of the Graduate School ________________ _________________ David P. Janos, Ph.D. Leonel da S. L. Sternberg, Ph.D. Associate Professor of Biology Professor of Biology ________________ Josiane Le Corff, Ph.D. Associate Professor of Biology Institut National d'Horticulture et de Paysage COZZA, JOHN (Ph.D., Biology) Sex Expression in a Rainforest Understory Herb, (December 2008) Begonia urophylla. -

Eriophyoid Studies in Turkey: Review and Perspectives
BIOLOGICAL LETT. 2013, 50(1): 45–54 Available online at: http:/www.degruyter.com/view/j/biolet DOI: 10.2478/biolet-2013-0005 Eriophyoid studies in Turkey: review and perspectives EVSEL DENIZHAN1, WIKTORIA SZYDŁO2 and ANNA SKORACKA2 1Department of Plant Protection, University of Yüzüncü Yil, Agricultural Faculty, 65080 Van, Turkey 2Adam Mickiewicz University, Institute of Environmental Biology, Department of Animal Taxonomy and Ecology, Umultowska 89, 60-687 Poznań, Poland Corresponding author: Wiktoria Szydło, [email protected] (Received on 31 January 2012; Accepted on 22 May 2013) Abstract: Although the geographical location and botanical history of Turkey make the country a perfect place for a potentially rich diversity of eriophyoid mites, little is known about the Turkish eriophyoid fauna. The current paper is a brief review of the existing records of eriophyoid mites found so far in Turkey, with additional information on 6 grass-associated eriophyoid species recorded recently. The 134 eriophyoid species collected in Turkey come from only ca. 1.2% of all Turkish plant species. The role of collecting ecological and molecular data and studying economically significant eriophyoid mites species in this area is particularly stressed. Keywords: Eriophyoidea, new records, faunistic, Turkey INTRODUCTION The superfamily Eriophyoidea is one of the largest groups of plant-feeding ar- thropods, which includes plant parasites of great economic significance (lindQuist et al. 1996). Their special importance is due to their potential influence on crops, orchards, and forests all over the world. They are in fact major pests causing signifi- cant economic losses in agriculturally important plants (duso et al. 2010). They can cause various plant deformations and abnormalities, e.g. -

Comparative Anatomical Study on Leaves of Three Euphorbia L. Species Rodica Bercu & Dan Răzvan Popoviciu
© Landesmuseum für Kärnten; download www.landesmuseum.ktn.gv.at/wulfenia; www.zobodat.at Wulfenia 22 (2015): 271–276 Mitteilungen des Kärntner Botanikzentrums Klagenfurt Comparative anatomical study on leaves of three Euphorbia L. species Rodica Bercu & Dan Răzvan Popoviciu Summary: The paper presents a comparative study on the leaf structure of three species belonging to Euphorbiaceae: Euphorbia myrsinites, E. nicaeensis subsp. dobrogensis and E. seguieriana. Anatomically, the leaves of the three species are relatively similar in their basic structure. However, differences appear regarding epidermal cells and cuticular papillae, mesophyll, density of stomata and type of stomatal complex, laticifers and development of the vascular system. Keywords: comparative anatomy, laticifers, leaves, stomata, Euphorbia Euphorbiaceae is the sixth largest plant family comprising 322 genera and about 8910 species, most common in the humid tropical and subtropical regions of both hemispheres (Webster 1987). Many species are commonly known as spurges (Radcliffe-Smith 1987 ). Euphorbia myrsinites L., also known as myrtle spurge, creeping spurge or donkey tail, is a perennial, herbaceous plant with sprawling stems growing up to 20 – 40 cm. The leaves are spirally arranged, fleshy, pale, glaucous, blueish-green, 1–2 cm long. The flowers are inconspicuous, but surrounded by bright sulphurous-yellow bracts (tinged red). They are flowering in spring. In the Romanian flora,Euphorbia myrsinites is considered as an endangered species (Dihoru & Negrean 2009). Euphorbia nicaeensis subsp. dobrogensis (Prodán) Kuzmanov is a herbaceous perennial, pale green, with hairless stems growing up to 40 cm, densely-foliated and branched at the top. The leaves are spirally arranged, 30 – 60 mm long and 15 –20 mm wide, elliptical in shape, obtuse on top and shortly mucronated. -
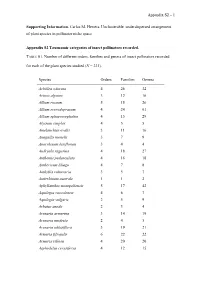
1 Supporting Information. Carlos M. Herrera. Unclusterable
Appendix S2 – 1 Supporting Information. Carlos M. Herrera. Unclusterable, underdispersed arrangement of plant species in pollinator niche space. Appendix S2 Taxonomic categories of insect pollinators recorded. TABLE S1. Number of different orders, families and genera of insect pollinators recorded for each of the plant species studied (N = 221). Species Orders Families Genera Achillea odorata 4 26 32 Acinos alpinus 3 12 16 Allium roseum 5 15 26 Allium scorodoprasum 4 24 61 Allium sphaerocephalon 4 15 29 Alyssum simplex 4 5 5 Amelanchier ovalis 3 11 16 Anagallis monelli 3 7 9 Anarrhinum laxiflorum 3 4 4 Andryala ragusina 4 18 27 Anthemis pedunculata 4 16 18 Anthericum liliago 4 7 8 Anthyllis vulneraria 3 5 7 Antirrhinum australe 1 1 2 Aphyllanthes monspeliensis 5 17 42 Aquilegia cazorlensis 4 6 7 Aquilegia vulgaris 2 5 9 Arbutus unedo 2 3 4 Arenaria armerina 3 14 19 Arenaria modesta 2 4 5 Arenaria obtusiflora 3 19 21 Armeria filicaulis 6 22 22 Armeria villosa 4 20 20 Asphodelus cerasiferus 4 12 15 Appendix S2 – 2 Astragalus bourgaeanus 1 3 3 Astragalus incanus 2 2 3 Atropa baetica 2 4 5 Bellis perennis 5 16 15 Bellis sylvestris 4 13 12 Berberis hispanica 3 17 23 Biscutella laxa 4 11 12 Calamintha nepeta 3 8 11 Campanula dieckii 4 14 17 Campanula mollis 3 5 5 Carduncellus monspelliensium 4 17 27 Carduus platypus 3 10 17 Carduus tenuiflorus 4 14 20 Carlina hispanica 4 13 37 Carlina racemosa 4 7 21 Catananche caerulea 4 17 32 Centaurea calcitrapa 4 10 30 Centaurea graminifolia 4 13 21 Cerastium gibraltaricum 4 11 12 Chaenorhinum macropodum -

Anatomical Characteristics and Antioxidant Properties of Euphorbia Nicaeensis Ssp
Cent. Eur. J. Biol. • 4(2) • 2009 • 214–223 DOI: 10.2478/s11535-009-0003-7 Central European Journal of Biology Anatomical characteristics and antioxidant properties of Euphorbia nicaeensis ssp. glareosa Research Article Jadranka Luković1, Djordje Malenčić2, Lana Zorić1*, Biljana Kiprovski1, Ljiljana Merkulov1, Pal Boža1 1Department of Biology and Ecology, Faculty of Sciences, University of Novi Sad, Novi Sad 21000, Serbia 2Faculty of Agriculture, University of Novi Sad, Novi Sad 21000, Serbia Received 23 September 2008; Accepted 8 January 2009 Abstract: Anatomical analyses found that leaves of Euphorbia nicaeensis ssp. glareosa are isolateral, amphistomatous, with two layers of palisade cells on the adaxial and one on the abaxial side. Laticifers are present by vascular bundles, in palisade and spongy tissue. Stem laticifers are located in the pericyclic ring, adjacent to the phloem, in cylinder parenchyma and medullar rays. The structure of pleiochasium and dichasium peduncle is similar to the stem structure. Plants from typical steppe habitat show more xeromorphic features. Phytochemical screening of extracts showed presence of catecholes, flavonoids, tannins, saponins, free quinone derivatives and absence of anthocyanins, leucoanthocyanins, alkaloids, steroid compounds and essential oils. Our results showed that the .- . examined taxon was partially susceptible to the action of reactive oxygen species, such as O2 and OH. The higher quantities of ROS thus provoked an antioxidative response from the plant, both in an enzymatic and non-enzymatic manner. Stable anatomical structure, presence and distribution of laticifers and effective antioxidant properties when exposed to ROS, make Euphorbia nicaeensis subsp. glareosa potentially interesting for further pharmaceutical and phytochemical examinations. Keywords: Anatomy • Antioxidant activity • Euphorbia • E. -

Annual Plant Reviews, Flowering and Its Manipulation
Flowering and its Manipulation Edited by CHARLES AINSWORTH Department of Agricultural Sciences Imperial College Wye Campus Ashford Kent UK Flowering and its Manipulation Annual Plant Reviews A series for researchers and postgraduates in the plant sciences. Each volume in this series focuses on a theme of topical importance and emphasis is placed on rapid publication. Editorial Board: Professor Jeremy A. Roberts (Editor-in-Chief), Plant Science Division, School of Biosciences, University of Nottingham, Sutton Bonington Campus, Loughborough, Leicestershire, LE12 5RD, UK; Dr David Evans, School of Biological and Molecular Sci- ences, Oxford Brookes University, Headington, Oxford, OX3 0BP; Professor Hidemasa Imaseki, Obata-Minami 2419, Moriyama-ku, Nagoya 463, Japan; Dr Michael T. McManus, Institute of Molecular BioSciences, Massey University, Palmerston North, New Zealand; Dr Jocelyn K.C. Rose, Department of Plant Biology, Cornell University, Ithaca, New York 14853, USA. Titles in the series: 1. Arabidopsis Edited by M. Anderson and J.A. Roberts 2. Biochemistry of Plant Secondary Metabolism Edited by M. Wink 3. Functions of Plant Secondary Metabolites and their Exploitation in Biotechnology Edited by M. Wink 4. Molecular Plant Pathology Edited by M. Dickinson and J. Beynon 5. Vacuolar Compartments Edited by D.G. Robinson and J.C. Rogers 6. Plant Reproduction Edited by S.D. O’Neill and J.A. Roberts 7. Protein–Protein Interactions in Plant Biology Edited by M.T. McManus, W.A. Laing and A.C. Allan 8. The Plant Cell Wall Edited by J.K.C. Rose 9. The Golgi Apparatus and the Plant Secretory Pathway Edited by D.G. Robinson 10. The Plant Cytoskeleton in Cell Differentiation and Development Edited by P.J. -

Flowers of Italy's Gargano Peninsula
Flowers of Italy's Gargano Peninsula Naturetrek Tour Report 15 - 22 April 2019 Ophrys scolopax ssp cornuta Orchis pauciflora Ophrys tenthredinifera Alpine Swift at Peschici Glanville Fritillary Report and images by Andrew Cleave Naturetrek Mingledown Barn Wolf's Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk Tour Report Flowers of Italy's Gargano Peninsula Tour participants: Andrew Cleave & Andrew Bray (leaders) with nine Naturetrek clients. Summary We managed to fit plenty of sites and lots of plants into our week in the “Orchid Capital of Europe”, visiting a good variety of plant-rich habitats ranging from breezy coastal salt marshes and limestone sea cliffs to typical Mediterranean stony hillsides, olive groves and shady woodlands. The orchids did not fail to delight, and we found a superb selection of species during the week. The non-orchid flora was equally attractive with showy displays of Narcissi, Wild Tulips, Anemones and Irises, and plenty of the usual Mediterranean species in all the areas we visited. We were fortunate in that most of the locations we looked at were almost deserted and we were able to explore beautiful woodlands and open glades with only bird-song and cow-bells to be heard. Butterflies were spotted at most of the sites we visited, and there were plenty of other interesting insects to be found as well. Although we spent most of our time looking down at the plants, we did manage to produce a very good bird list with some exciting coastal species seen, as well as the more usual Mediterranean birds of stony hillsides and dark forests. -

PUBLICATION LIST Božo Frajman
PUBLICATION LIST Božo Frajman Scientific articles in SCI journals 1. Frajman, B., Rabeler, R. K. (2006) Proposal to conserve the name Heliosperma against Ixoca (Caryophyllaceae, Sileneae). Taxon 55: 807–808. 2. Frajman, B., Oxelman, B. (2007) Reticulate phylogenetics and phytogeographical structure of Heliosperma (Sileneae, Caryophyllaceae) inferred from chloroplast and nuclear DNA sequences. Molecular Phylogenetics and Evolution 43: 140–155. 3. Manel, S., Berthoud, F., Bellemain, E., Gaudeul, M., Luikart, G., Swenson, J. E., Waits, L. P., Taberlet, P., & Intrabiodiv-Consortium1 (2007) A new individual-based spatial approach for identifying genetic discontinuities in natural populations. Molecular Ecology, 16: 2031–2043. 4. Ehrich, D., Gaudeul, M., Assefa, A., Koch, M. A., Mummenhof, K., Nemomissa, S., Intrabiodiv- Consortium1, & Brochmann, C. (2007) Genetic consequences of Pleistocene range shifts: Contrast between the Arctic, the Alps and the East African mountains. Molecular Ecology, 16: 2542–2559. 5. Frajman, B. (2007) Proposal to reject the name Cucubalus quadrifidus (Heliosperma quadrifidum, Silene quadrifida) (Caryophyllaceae, Sileneae). Taxon 56: 260–261. 6. Frajman, B., Schönswetter, P (2008) Notes on some rare Orobanche and Phelipanche species (Orobanchaceae) in Croatia. Acta Botanica Croatica 67: 103-107. 7. Schneeweiss, G. M., Frajman, B., Dakskobler, I. (2009) Orobanche lycoctoni Rhiner (Orobanchaceae), a poorly known species of the Central European flora. Candollea (Genève), 64: 91–99. 8. Frajman, B., Eggens, F., Oxelman, B. (2009) Hybrid origins and homoploid reticulate evolution within Heliosperma (Sileneae, Caryophyllaceae)-a multigene phylogenetic approach with relative dating. Systematic Biology 58: 328–345. 9. Frajman, B., Schneeweiss, G. M. (2009) A campanulaceous fate : the albanian stenoendemic Asyneuma comosiforme in fact belongs to isophyllous Campanula. -

An Inventory of Vascular Plants Endemic to Italy
Phytotaxa 168 (1): 001–075 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2014 Magnolia Press Monograph ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.168.1.1 PHYTOTAXA 168 An inventory of vascular plants endemic to Italy LORENZO PERUZZI1*, FABIO CONTI2 & FABRIZIO BARTOLUCCI2 1Dipartimento di Biologia, Unità di Botanica, Università di Pisa, Via Luca Ghini 13, 56126, Pisa, Italy; e-mail [email protected] 2Scuola di Scienze Ambientali, Università di Camerino – Centro Ricerche Floristiche dell’Appennino, Parco Nazionale del Gran Sasso e Monti della Laga, San Colombo, 67021 Barisciano (L'Aquila); e-mail [email protected]; [email protected] *author for correspondence Magnolia Press Auckland, New Zealand Accepted by Alex Monro: 12 Apr. 2014; published: 16 May 2014 1 Peruzzi et al. An inventory of vascular plants endemic to Italy (Phytotaxa 168) 75 pp.; 30 cm. 16 May 2014 ISBN 978-1-77557-378-4 (paperback) ISBN 978-1-77557-379-1 (Online edition) FIRST PUBLISHED IN 2014 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/phytotaxa/ © 2014 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use.