Hylotelephium Spectabile, a New Host for Carnation Tortrix Moth (Cacoecimorpha Pronubana) and Molecular Characterization in Greece
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Numerical Taxonomy of the Genus Rosularia (Dc.) Stapf from Pakistan and Kashmir
Pak. J. Bot., 44(1): 349-354, 2012. A NUMERICAL TAXONOMY OF THE GENUS ROSULARIA (DC.) STAPF FROM PAKISTAN AND KASHMIR GHULAM RASOOL SARWAR* AND MUHAMMAD QAISER Centre for Plant Conservation, University of Karachi, Karachi-75270, Pakistan Federal Urdu University of Arts, Science and Technology, Gulshan-e-Iqbal, Karachi, Pakistan Abstract Numerical analysis of the taxa belonging to the genus Rosularia (DC.) Stapf was carried out to find out their phenetic relationship. Data from different disciplines viz. general, pollen and seed morphology, chemistry and distribution pattern were used. As a result of cluster analysis two distinct groups are formed. Out of which one group consists of R. sedoides (Decne.) H. Ohba and R. alpestris A. Boriss. while other group comprises R. adenotricha (Wall. ex Edgew.) Jansson ssp. adenotricha , R. adenotricha ssp. chitralica, G.R. Sarwar, R. rosulata (Edgew.) H. Ohba and R. viguieri (Raym.-Hamet ex Frod.) G.R. Sarwar. Distribution maps of all the taxa, along with key to the taxa are also presented. Introduction studied the genus Rosularia and indicated that the genus is polyphyletic. Mayuzumi & Ohba (2004) analyzed the Rosularia is a small genus composed of 28 species, relationships within the genus Rosularia. According to distributed in arid or semiarid regions ranging from N. different workers Rosularia is polyphyletic. Africa to C. Asia through E. Mediterranean (Mabberley, There are no reports on numerical studies of 2008). Some of the taxa of Rosularia are in general Crassulaceae except the genus Sedum from Pakistan cultivation and several have great appeal due to their (Sarwar & Qaiser, 2011). The primary aim of this study is extraordinarily regular rosettes on the leaf colouring in to analyze diagnostic value of morphological characters in various seasons. -

FLOWERS Herbaceous Perennials No
G A R D E N I N G S E R I E S FLOWERS Herbaceous Perennials no. 7.405 by M. Meehan, J.E. Klett and R.A. Cox 1 An ever-expanding palette of perennials lets home gardeners create showy collections of herbaceous perennials. Quick Facts... Under normal growing conditions, perennials live many years, dying back to the ground each winter. They quickly establish These herbaceous perennials themselves in a few growing seasons and create a are best adapted for Colorado’s backbone for the flower garden. lower elevations. Plants vary in flower color, bloom time, height, foliage texture and environmental Herbaceous perennials differ requirements. Environmental requirements include in bloom period, flower color, sun exposure, soil conditions and water needs. height, foliage texture and The key to a successful perennial garden is to environmental requirements. choose plants whose requirements match your site’s conditions. Environmental requirements Table 1 lists perennials adapted to the broad include sun and wind exposure range of growing conditions in Colorado’s lower or lack of it, soil conditions and elevations. Many also do well at higher elevations, but water needs. for a more specific listing of higher elevation perennials, see fact sheet 7.406, Flowers for Mountain Communities. Matching the perennial plant to More information on design and maintenance of perennial the site conditions produces a gardens can be found in 7.402, Perennial Gardening. Also see 7.840, Vegetable Garden: Soil Management and Fertilization. successful perennial garden. Key to Table 1: a only most common cultivars are listed. b Not Important. -

Bright Spots Plant Material Location
Bright Spots A SELF GUIDED WALKING TOUR September 13, 2017 Did you know…? Usually when we think of azaleas we think of Spring, but there are some beauties abloom in the garden right now. These are reblooming azaleas. They have already produced Spring flowers for Virginia’s Garden Week, and now they are contributing to Fall color. Many of these plants are patented under the name Encore® azalea. They first became available in the late 1990s, the work of Robert E. “Buddy” Lee, a plant breeder and nurseryman from Louisiana. This group derives from a cross between spring blooming azaleas and Rhododendron oldhamii “Fourth of July”. The garden’s Plant Explorer database lists 22 varieties of Encore® azaleas. Many have the word “Autumn” in their cultivar name. Several are in bloom now, including ‘Autumn Royalty’ and ‘Autumn Sundance’ listed below. Not much published material is available on these rebloomers, but the article by Will Ferrell (not the actor) in The Azalean (below) is a knowledgeable evaluation of the merits of many specific cultivars. Besides the Encore® series there are at least two other patented reblooming lines: ReBLOOM™ Azaleas by breeder Bob Head and Bloom-a-thon from Monrovia. To sow confusion, I must note that the Azalea ‘August to Frost’ with its bright white blossoms (along the Flagler walk) is not considered a rebloomer. It was hybridized by M. B. Matlack in 1940, possibly a cross between R. mucronatum var. mucronatum and an unknown species. Sources: http://www.encoreazalea.com/; “Personal Observations on Encore® Azaleas in a Zone 7 Garden”, Will Ferrell, The Azalean, Fall 2013, pp. -

The Role of Starch in the Day/Night Re-Programming of Stomata in Plants with Crassulacean Acid Metabolism Natalia Hurtado Castan
The role of starch in the day/night re-programming of stomata in plants with Crassulacean Acid Metabolism Natalia Hurtado Castano Doctor of Philosophy School of Natural and Environmental Sciences February 2020 Declaration This thesis is submitted to Newcastle University for the degree of Doctor of Philosophy. The research detailed within was performed between the years 2015-2019 and it was supervised by Professor Anne Borland and Professor Jeremy Barnes. I certify that none of the material offered in this thesis has been previously submitted by me for a degree or any other qualification at this or any other university. Natalia Hurtado Castano ii Abstract Crassulacean acid metabolism (CAM) is a specialised type of photosynthesis characterised by the unique inverted stomatal rhythm, which increases water use efficiency (WUE) and enhances the potential for sustainable biomass production in warmer and drier conditions. Starch turnover in the mesophyll of CAM species supports nocturnal CO2 assimilation and CAM activity. In C3 plants, starch metabolism has been reported to play an important role in determining stomatal behaviour; in this case, guard cell starch degradation is triggered by blue light, producing osmolytes that promotes stomatal opening. Based on the importance of starch and the little knowledge regarding CAM stomatal behaviour, this study tested the hypothesis that starch metabolism has been re-programmed in CAM plants to enable nocturnal stomatal opening, by using biochemical and genetic characterisation of wild type and RNAi lines with curtailed starch metabolism in the constitutive CAM species Kalanchoë fedtschenkoi. Measurements of guard cell starch content over 24 hours in wild type plants of K. -

Lepidoptera: Tortricidae: Tortricinae) and Evolutionary Correlates of Novel Secondary Sexual Structures
Zootaxa 3729 (1): 001–062 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3729.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:CA0C1355-FF3E-4C67-8F48-544B2166AF2A ZOOTAXA 3729 Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures JASON J. DOMBROSKIE1,2,3 & FELIX A. H. SPERLING2 1Cornell University, Comstock Hall, Department of Entomology, Ithaca, NY, USA, 14853-2601. E-mail: [email protected] 2Department of Biological Sciences, University of Alberta, Edmonton, Canada, T6G 2E9 3Corresponding author Magnolia Press Auckland, New Zealand Accepted by J. Brown: 2 Sept. 2013; published: 25 Oct. 2013 Licensed under a Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0 JASON J. DOMBROSKIE & FELIX A. H. SPERLING Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures (Zootaxa 3729) 62 pp.; 30 cm. 25 Oct. 2013 ISBN 978-1-77557-288-6 (paperback) ISBN 978-1-77557-289-3 (Online edition) FIRST PUBLISHED IN 2013 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2013 Magnolia Press 2 · Zootaxa 3729 (1) © 2013 Magnolia Press DOMBROSKIE & SPERLING Table of contents Abstract . 3 Material and methods . 6 Results . 18 Discussion . 23 Conclusions . 33 Acknowledgements . 33 Literature cited . 34 APPENDIX 1. 38 APPENDIX 2. 44 Additional References for Appendices 1 & 2 . 49 APPENDIX 3. 51 APPENDIX 4. 52 APPENDIX 5. -
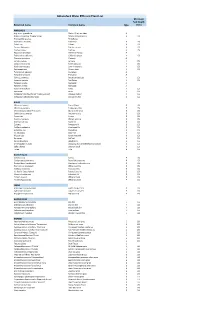
Abbotsford Water Efficient Plant List Minimum Soil Depth Botanical Name Common Name Type (Mm)
Abbotsford Water Efficient Plant List Minimum Soil Depth Botanical name Common name type (mm) ANNUALS Argemone grandifolia Statice/Sea Lavendar A Begonia x hybrida 'Dragon Wings' Dragon Wing begonia A 150 Bracteantha species Strawflower Calendula officinalis Calendula A 150 Coleus ssp. Coleus A 150 Cosmos bipinnatus Garden cosmos A 150 Cuphea llavea Cuphea A 150 Dyssocua tenuiloba Dahlberg Daisey A Eschscholzia californica California poppy A 150 Gazania spendens Gazania A Lantana camara Lantana A 150 Lobularia maritima Sweet alyssum A 150 Nigella damascena Love-in-the-mist A 150 Oesteopermum African daisy A 150 Pelargonium species Geranium A Portulaca oleracea Portulaca A Salvia guaranitica Anise-scented sage A 150 Scaevola aemula Fan flower A 150 Targetes erecta Marogold A Targetes erecta Marogold A Viola x wittrockiana Pansy A 150 Zinnia ssp. Zinnia A 150 Verbascum bombyciferum 'Arctic Summer' Broussa mullein A 150 Verbascum phlomoides 'Spica' Orange mullein A 150 BULBS Allium christophii Star of Persia B 150 Allium karataviense Turkestan onion B 150 Chionodoxa forbesii 'Pink Giant' Glory of the snow B 150 Colchicum autumnale Autumn crocus B 150 Crocus ssp. Crocus B 150 Eranthis hyemalis Winter aconite B 150 Erythronium ssp. Fawn lily B 150 Eucomis Pineapple lily B 150 Fritillaria meleagris Checkered lily B 150 Galanthus ssp. Snowdrop B 150 Iris reticulata Dwarf iris B 150 Muscari ssp. Grape hyacinth B 150 Narcissus Daffodil B 150 Nerine bowdenii Bowden lily B 150 Ornithogalum nutans Drooping Star of Bethlehem/Silverbells B 150 Scilla -

CRASSULACEAE 景天科 Jing Tian Ke Fu Kunjun (傅坤俊 Fu Kun-Tsun)1; Hideaki Ohba 2 Herbs, Subshrubs, Or Shrubs
Flora of China 8: 202–268. 2001. CRASSULACEAE 景天科 jing tian ke Fu Kunjun (傅坤俊 Fu Kun-tsun)1; Hideaki Ohba 2 Herbs, subshrubs, or shrubs. Stems mostly fleshy. Leaves alternate, opposite, or verticillate, usually simple; stipules absent; leaf blade entire or slightly incised, rarely lobed or imparipinnate. Inflorescences terminal or axillary, cymose, corymbiform, spiculate, racemose, paniculate, or sometimes reduced to a solitary flower. Flowers usually bisexual, sometimes unisexual in Rhodiola (when plants dioecious or rarely gynodioecious), actinomorphic, (3 or)4– 6(–30)-merous. Sepals almost free or basally connate, persistent. Petals free or connate. Stamens as many as petals in 1 series or 2 × as many in 2 series. Nectar scales at or near base of carpels. Follicles sometimes fewer than sepals, free or basally connate, erect or spreading, membranous or leathery, 1- to many seeded. Seeds small; endosperm scanty or not developed. About 35 genera and over 1500 species: Africa, America, Asia, Europe; 13 genera (two endemic, one introduced) and 233 species (129 endemic, one introduced) in China. Some species of Crassulaceae are cultivated as ornamentals and/or used medicinally. Fu Shu-hsia & Fu Kun-tsun. 1984. Crassulaceae. In: Fu Shu-hsia & Fu Kun-tsun, eds., Fl. Reipubl. Popularis Sin. 34(1): 31–220. 1a. Stamens in 1 series, usually as many as petals; flowers always bisexual. 2a. Leaves always opposite, joined to form a basal sheath; inflorescences axillary, often shorter than subtending leaf; plants not developing enlarged rootstock ................................................................ 1. Tillaea 2b. Leaves alternate, occasionally opposite proximally; inflorescence terminal, often very large; plants sometimes developing enlarged, perennial rootstock. -

Additions, Deletions and Corrections to An
Bulletin of the Irish Biogeographical Society No. 36 (2012) ADDITIONS, DELETIONS AND CORRECTIONS TO AN ANNOTATED CHECKLIST OF THE IRISH BUTTERFLIES AND MOTHS (LEPIDOPTERA) WITH A CONCISE CHECKLIST OF IRISH SPECIES AND ELACHISTA BIATOMELLA (STAINTON, 1848) NEW TO IRELAND K. G. M. Bond1 and J. P. O’Connor2 1Department of Zoology and Animal Ecology, School of BEES, University College Cork, Distillery Fields, North Mall, Cork, Ireland. e-mail: <[email protected]> 2Emeritus Entomologist, National Museum of Ireland, Kildare Street, Dublin 2, Ireland. Abstract Additions, deletions and corrections are made to the Irish checklist of butterflies and moths (Lepidoptera). Elachista biatomella (Stainton, 1848) is added to the Irish list. The total number of confirmed Irish species of Lepidoptera now stands at 1480. Key words: Lepidoptera, additions, deletions, corrections, Irish list, Elachista biatomella Introduction Bond, Nash and O’Connor (2006) provided a checklist of the Irish Lepidoptera. Since its publication, many new discoveries have been made and are reported here. In addition, several deletions have been made. A concise and updated checklist is provided. The following abbreviations are used in the text: BM(NH) – The Natural History Museum, London; NMINH – National Museum of Ireland, Natural History, Dublin. The total number of confirmed Irish species now stands at 1480, an addition of 68 since Bond et al. (2006). Taxonomic arrangement As a result of recent systematic research, it has been necessary to replace the arrangement familiar to British and Irish Lepidopterists by the Fauna Europaea [FE] system used by Karsholt 60 Bulletin of the Irish Biogeographical Society No. 36 (2012) and Razowski, which is widely used in continental Europe. -

The Entomologist's Record and Journal of Variation
M DC, — _ CO ^. E CO iliSNrNVINOSHilWS' S3ldVyan~LIBRARlES*"SMITHS0N!AN~lNSTITUTl0N N' oCO z to Z (/>*Z COZ ^RIES SMITHSONIAN_INSTITUTlON NOIiniIiSNI_NVINOSHllWS S3ldVaan_L: iiiSNi'^NviNOSHiiNS S3iavyan libraries Smithsonian institution N( — > Z r- 2 r" Z 2to LI ^R I ES^'SMITHSONIAN INSTITUTlON'"NOIini!iSNI~NVINOSHilVMS' S3 I b VM 8 11 w </» z z z n g ^^ liiiSNi NviNOSHims S3iyvyan libraries Smithsonian institution N' 2><^ =: to =: t/J t/i </> Z _J Z -I ARIES SMITHSONIAN INSTITUTION NOIiniliSNI NVINOSHilWS SSIdVyan L — — </> — to >'. ± CO uiiSNi NViNosHiiws S3iyvaan libraries Smithsonian institution n CO <fi Z "ZL ~,f. 2 .V ^ oCO 0r Vo^^c>/ - -^^r- - 2 ^ > ^^^^— i ^ > CO z to * z to * z ARIES SMITHSONIAN INSTITUTION NOIinillSNl NVINOSHllWS S3iaVdan L to 2 ^ '^ ^ z "^ O v.- - NiOmst^liS^> Q Z * -J Z I ID DAD I re CH^ITUCnMIAM IMOTtTIITinM / c. — t" — (/) \ Z fj. Nl NVINOSHIIINS S3 I M Vd I 8 H L B R AR I ES, SMITHSONlAN~INSTITUTION NOIlfl :S^SMITHS0NIAN_ INSTITUTION N0liniliSNI__NIVIN0SHillMs'^S3 I 8 VM 8 nf LI B R, ^Jl"!NVINOSHimS^S3iavyan"'LIBRARIES^SMITHS0NIAN~'lNSTITUTI0N^NOIin L '~^' ^ [I ^ d 2 OJ .^ . ° /<SS^ CD /<dSi^ 2 .^^^. ro /l^2l^!^ 2 /<^ > ^'^^ ^ ..... ^ - m x^^osvAVix ^' m S SMITHSONIAN INSTITUTION — NOIlfliliSNrNVINOSHimS^SS iyvyan~LIBR/ S "^ ^ ^ c/> z 2 O _ Xto Iz JI_NVIN0SH1I1/MS^S3 I a Vd a n^LI B RAR I ES'^SMITHSONIAN JNSTITUTION "^NOlin Z -I 2 _j 2 _j S SMITHSONIAN INSTITUTION NOIinillSNI NVINOSHilWS S3iyVaan LI BR/ 2: r- — 2 r- z NVINOSHiltNS ^1 S3 I MVy I 8 n~L B R AR I Es'^SMITHSONIAN'iNSTITUTIOn'^ NOlin ^^^>^ CO z w • z i ^^ > ^ s smithsonian_institution NoiiniiiSNi to NviNosHiiws'^ss I dVH a n^Li br; <n / .* -5^ \^A DO « ^\t PUBLISHED BI-MONTHLY ENTOMOLOGIST'S RECORD AND Journal of Variation Edited by P.A. -

[email protected]
Import Health Standard Commodity Sub-class: Fresh Fruit/Vegetables Korean pear, Pyrus pyrifolia from the Republic of Korea ISSUED Issued pursuant to Section 22 of the Biosecurity Act 1993 Date Issued: 29 July 1999 1 NEW ZEALAND NATIONAL PLANT PROTECTION ORGANISATION The New Zealand national plant protection organisation is the Ministry of Agriculture and Forestry and as such, all communication should be addressed to: Chief Plants Officer Ministry of Agriculture and Forestry PO Box 2526 Wellington NEW ZEALAND Fax: 64-4-474 4240 E-mail: [email protected] http://www.maf.govt.nz 2 GENERAL CONDITIONS FOR ALL PLANT PRODUCTS All plants and plant products are PROHIBITED entry into New Zealand, unless an import health standard has been issued in accordance with Section 22 of the Biosecurity Act 1993. Should prohibited plants or plant products be intercepted by the New Zealand Ministry of Agriculture and Forestry, the importer will be offered the option of reshipment or destruction of the consignment. The national plant protection organisation of the exporting country is requested to inform the New Zealand Ministry of Agriculture and Forestry of any change in its address. The national plant protection organisation of the exporting country is required to inform the New Zealand Ministry of Agriculture and Forestry of any newly recorded organisms which may infest/infect any commodity approved for export to New Zealand. Pursuant to the Hazardous Substances and New Organisms Act 1996, proposals for the deliberate introduction of new organisms (including genetically modified organisms) as defined by the Act IHS Fresh Fruit/Vegetables. Korean pear, Pyrus pyrifolia from the Republic of Korea (Biosecurity Act 1993) ISSUED: 29 July 1999 Page 1 of 16 should be referred to: Manager, Operations Environment Risk Management Authority PO Box 131 Wellington NEW ZEALAND Also note: In order to meet the Environmental Risk Management Authority's requirements the scientific name (i.e. -

292-9999 Fax: (949) 574-8355
TEL: (949) 292-9999 KBD NURSERY EMAIL: [email protected] FAX: (949) 574-8355 11/20/2017 DECO SUCCULENT MIX 2.5" 4" 6 MIX 8 BOWL T= TOP SHELF SUCCULENT MIX FLATS 400 F= Flowering T SUCCULENT MIX TERRA COTA 4" (SEE PHOTO) NEW 100* HANGING BASKETS 6" 8" T Sedum Donkey Tails *1000 T Senecio Jacobsenii *500 Senecio String of Banannas T Senecio String of Pearls *50 AEONIUMS 4" Quarts 6" 8/10" 1 gal. 2/5g 15g 25g Aeonium Arboreum 100 Aeonium Atropurpureum Aeonium Crush Aeonium Cabernet 100 Aeonium Canariense Aeonium Cyclops 100 Aeoinium Haworthia 100 25 T Aeonium Lily Pad NEW 1000 *500 Aeonium Purple Blast Aeonium Sunburst Aeonium Tabuliforme-Mint Saucer 100 Aeonium Tricolor Kiwi 1000 Aeonium Urbicum- Salad Bowl 500 250 Aeonium Zwartkin Aeonium Zwartkop x Tabliforme Aeonium Zwartkop-Black Rose 100 ALOES 4" Quarts 6" 8/10" 1g 2/5g 15g 25g Aloe Aristata-Lace Aloe 200 200 Aloe Bainesii-Aloe Tree Aloe Blue Elf 1,000 250 Aloe Brevifolia-Short Leaf Aloe 200 Aloe Cameronii Aloe Ciliaris-Climbing Aloe 80 T Aloe Christmas Carol *500 100 Aloe Coral-Striata 1,000 3,000 100 Aloe Crosby Aloe Cynthia Giddy 1,000 800 Aloe Delta Lights 100 50 Aloe Dorotheae-Red Aloe Aloe Fang Aloe Ferox 100 36 Aloe Grassy Lassie 100 100 Aloe Maculata Aloe Noblis-Gold Tooth Aloe 1,000 T Aloe Pink Blush *500 30 Aloe Plicatilis-Fan Aloe 1 TEL: (949) 292-9999 KBD NURSERY EMAIL: [email protected] FAX: (949) 574-8355 11/20/2017 ALOES 4" Quarts 6" 8/10" 1g 2/5g 15g 25g Aloe Rooikoppie 500 500 F Aloe Rubroviolence *50 Aloe Traskii Aloe Torch-Arborescens 1,000 500 100 40 Aloe Variegata-Tiger -

The Insect Database in Dokdo, Korea: an Updated Version in 2020
Biodiversity Data Journal 9: e62011 doi: 10.3897/BDJ.9.e62011 Data Paper The Insect database in Dokdo, Korea: An updated version in 2020 Jihun Ryu‡,§, Young-Kun Kim |, Sang Jae Suh|, Kwang Shik Choi‡,§,¶ ‡ School of Life Science, BK21 FOUR KNU Creative BioResearch Group, Kyungpook National University, Daegu, South Korea § Research Institute for Dok-do and Ulleung-do Island, Kyungpook National University, Daegu, South Korea | School of Applied Biosciences, Kyungpook National University, Daegu, South Korea ¶ Research Institute for Phylogenomics and Evolution, Kyungpook National University, Daegu, South Korea Corresponding author: Kwang Shik Choi ([email protected]) Academic editor: Paulo Borges Received: 14 Dec 2020 | Accepted: 20 Jan 2021 | Published: 26 Jan 2021 Citation: Ryu J, Kim Y-K, Suh SJ, Choi KS (2021) The Insect database in Dokdo, Korea: An updated version in 2020. Biodiversity Data Journal 9: e62011. https://doi.org/10.3897/BDJ.9.e62011 Abstract Background Dokdo, a group of islands near the East Coast of South Korea, comprises 89 small islands. These volcanic islands were created by an eruption that also led to the formation of the Ulleungdo Islands (located in the East Sea), which are approximately 87.525 km away from Dokdo. Dokdo is important for geopolitical reasons; however, because of certain barriers to investigation, such as weather and time constraints, knowledge of its insect fauna is limited compared to that of Ulleungdo. Until 2017, insect fauna on Dokdo included 10 orders, 74 families, 165 species and 23 undetermined species; subsequently, from 2018 to 2019, we discovered 23 previously unrecorded species and three undetermined species via an insect survey.