Attention and Olfactory Consciousness
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of Perceptual Load in Visual Awareness
BRAIN RESEARCH 1080 (2006) 91– 100 available at www.sciencedirect.com www.elsevier.com/locate/brainres Research Report The role of perceptual load in visual awareness Nilli Lavie⁎ Department of Psychology, University College London, Gower Street, London WC1E 6BT, UK ARTICLE INFO ABSTRACT Article history: Does awareness depend on attention? This is a fundamental issue for understanding the Accepted 6 October 2005 relationship of attention and awareness, yet previous research provided mixed results. Available online 18 January 2006 Here, I describe new research that shows that the effects of attention on awareness depend on the level of perceptual load in the attended task. Awareness reports in both Keywords: the inattentional blindness and change blindness paradigms were found to depend on the Attention extent to which an attended primary task loads attention. Neuroimaging results revealed Awareness the involvement of frontoparietal attention network in awareness and transcranial Load magnetic stimulation experiments confirmed a causal role for frontoparietal activity in Change blindness awareness. These results clarify the role of attention and associated frontoparietal Inattentional blindness activity in visual awareness within the framework of load theory of attention. Distracter © 2005 Published by Elsevier B.V. Neuroimaging TMS 1. Introduction from the task-relevant processing spills over involuntarily, resulting in the perception of irrelevant stimuli (late selection). Would focusing attention on a current task prevent intrusions -

Attention : Change Blin Dness and Inatt Entional Blindnes S R a Rensink, University of British Columbia, Vancouver, BC, Canada à 2009 Elsevier Inc
a0005 Attention : Change Blin dness and Inatt entional Blindnes s R A Rensink, University of British Columbia, Vancouver, BC, Canada ã 2009 Elsevier Inc. All rights reserved. Glossary is false – there are severe limits to what we can consciously experience in everyday life. Much of g0005 Change blindness – The failure to visually the evidence for this claim has come from two experience changes that are easily seen once phenomena: change blindness (CB) and inatten- noticed. This failure therefore cannot be due tional blindness (IB). to physical factors such as poor visibility; CB refers to the failure of an observer to visu- perceptual factors must be responsible. ally experience changes that are easily seen once Focused attention is believed to be necessary noticed. This can happen even if the changes are to see change, with change blindness large, constantly repeat, and the observer has been resulting if such attention is not allocated to informed that they will occur. A related phenome- the object at the moment it changes. non is IB – the failure to visually experience an g0010 Diffuse attention – A type of attention that is object or event when attention is directed else- spread out over large areas of space. It is where. For example, observers may fail to notice believed to be space-based rather than an unexpected object that enters their visual field, object-based. even if this object is large, appears for several g0015 Focused attention – A type of attention seconds, and has important consequences for the restricted to small spatial extents. It is selection of action. -
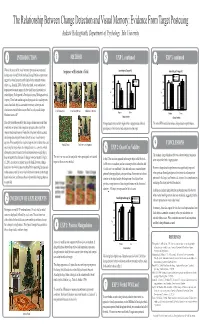
The Relationship Between Change Detection and Visual Memory: Evidence from Target Postcuing Andrew Hollingworth, Department of Psychology, Yale University
The Relationship Between Change Detection and Visual Memory: Evidence From Target Postcuing Andrew Hollingworth, Department of Psychology, Yale University 1 INTRODUCTION 3 METHOD 5 EXP 1, continued 8 EXP 3, continued What is the nature of the visual memory representation constructed Sequence of Events in a Trial Sensitivity to Change (A') Sensitivity to Change (A') during scene viewing? On the one hand, change blindness experiments 1.00 1.00 No Load suggest that visual memory may be limited to the currently attended Verbal Load 0.90 Postcue 0.90 A B C object (e.g., Rensink, 2000). On the other hand, recent studies have No postcue demonstrated accurate memory for the visual form of previously at- 0.80 0.80 A' A' tended objects (Hollingworth & Henderson, in press; Hollingworth et al., 0.70 0.70 in press). These latter studies support the proposal that visual represen- 0.60 tations from local objects accumulate in memory as the eyes and 0.60 0.880.77 0.89 0.73 0.890.87 0.85 0.91 attention are oriented within a scene. But if so, why would change 0.50 0.50 Rotation Token blindness occur at all? Rotation Token D E Change Condition Change Condition This study tested the possibility that change blindness may result from Change detection was reliably higher when a target postcue allowed The verbal WM load did not reduce change detection performance. constraints on retrieval and comparison processes rather than from participants to limit retrieval and comparison to the target. impoverished visual memory. Heretofore, it has been widely assumed that change detection performance directly reflects visual memory capacity. -

Top ^ Down Control of Visual Perception: Attention in Natural Vision
Perception, 2008, volume 37, pages 333 ^ 354 doi:10.1068/p5877 Top ^ down control of visual perception: Attention in natural vision Edmund T Rolls Department of Experimental Psychology, University of Oxford, South Parks Road, Oxford OX1 3UD, UK; e-mail: [email protected] Presented at Interdisciplinary Conference on Pre-Emptive Perception, Hanse Institute for Advanced Studies, Delmenhorst, Germany, 15 ^ 18 October 2005 Abstract. Top ^ down perceptual influences can bias (or pre-empt) perception. In natural scenes, the receptive fields of neurons in the inferior temporal visual cortex (IT) shrink to become close to the size of objects. This facilitates the read-out of information from the ventral visual system, because the information is primarily about the object at the fovea. Top ^ down attentional influences are much less evident in natural scenes than when objects are shown against blank backgrounds, though are still present. It is suggested that the reduced receptive-field size in natural scenes, and the effects of top ^ down attention contribute to change blindness. The receptive fields of IT neurons in complex scenes, though including the fovea, are frequently asymmetric around the fovea, and it is proposed that this is the solution the IT uses to represent multiple objects and their relative spatial positions in a scene. Networks that implement probabilistic decision-making are described, and it is suggested that, when in perceptual systems they take decisions (or `test hypotheses'), they influence lower-level networks to bias visual perception. Finally, it is shown that similar processes extend to systems involved in the processing of emotion-provoking sensory stimuli, in that word-level cognitive states provide top ^ down biasing that reaches as far down as the orbitofrontal cortex, where, at the first stage of affective representations, olfactory, taste, flavour, and touch processing is biased (or pre-empted) in humans. -

Human Olfaction: a Constant State of Change-Blindness
Exp Brain Res (2010) 205:13–29 DOI 10.1007/s00221-010-2348-6 REVIEW Human olfaction: a constant state of change-blindness Lee Sela · Noam Sobel Received: 18 January 2010 / Accepted: 21 June 2010 / Published online: 7 July 2010 © The Author(s) 2010. This article is published with open access at Springerlink.com Abstract Paradoxically, although humans have a superb Introduction sense of smell, they don’t trust their nose. Furthermore, although human odorant detection thresholds are very low, Mammalian olfaction is highly conserved (Ache and only unusually high odorant concentrations spontaneously Young 2005). Indeed, the human olfactory system is not shift our attention to olfaction. Here we suggest that this very diVerent from that of goats and guinipigs. That said, lack of olfactory awareness reXects the nature of olfactory the place of olfaction in human behavior is diVerent, and attention that is shaped by the spatial and temporal enve- seemingly largely diminished in comparison to most mam- lopes of olfaction. Regarding the spatial envelope, selective mals (Stevenson 2009a). In this review we will propose two attention is allocated in space. Humans direct an attentional mechanistic reasons for this state of aVairs. Beforehand, spotlight within spatial coordinates in both vision and audi- however, we will brieXy outline human olfactory neuro- tion. Human olfactory spatial abilities are minimal. Thus, anatomy, and human olfactory capabilities. with no olfactory space, there is no arena for olfactory selective attention. Regarding the temporal envelope, whereas vision and audition consist of nearly continuous Human olfactory neuroanatomy input, olfactory input is discreet, made of sniVs widely sep- arated in time. -

Detecting Changes in Real-World Objects: the Relationship Between
[Communicative & Integrative Biology 2:1, 1-3; January/February 2009]; ©2009Change Landes blindness Bioscience and visual long-term memory Article Addendum Detecting changes in real-world objects The relationship between visual long-term memory and change blindness Timothy F. Brady,1 Talia Konkle,1 Aude Oliva1 and George A. Alvarez2,* 1Department of Brain and Cognitive Sciences; Massachusetts Institute of Technology; Cambridge, Massachusetts USA; 2Department of Psychology; Harvard University; Cambridge, Massachusetts USA Key words: object recognition, gist, fidelity, change blindness, change detection A large body of literature has shown that observers often fail to showing storage of a large number of items in long-term memory notice significant changes in visual scenes, even when these changes (see refs. 10–12). In this experiment, participants viewed pictures happen right in front of their eyes. For instance, people often fail to of 2,500 categorically distinct objects one at a time for 3 seconds notice if their conversation partner is switched to another person, each, over the course of 5.5 hours. Afterwards, they were shown or if large background objects suddenly disappear.1,2 These ‘change pairs of images, and indicated which of the two they had seen. The blindness’ studies have led to the inference that the amount of previously viewed item could be paired with either an object from information we remember about each item in a visual scene may a novel category (a cup you saw versus a clock you never saw), an be quite low.1 However, in recent work we have demonstrated that object of the same basic level category (a cup you saw versus a similar long-term memory is capable of storing a massive number of visual but different cup), or the same object in a different state (the cup objects with significant detail about each item.3 In the present you saw empty versus the same cup with juice in it). -

What's That Smell-Journal
Southern Journal of Philosophy (2009) VLVII: 321-348. What’s That Smell? Clare Batty University of Kentucky In philosophical discussions of the secondary qualities, color has taken center stage. Smells, tastes, sounds, and feels have been treated, by and large, as mere accessories to colors. We are, as it is said, visual creatures. This, at least, has been the working assumption in the philosophy of perception and in those metaphysical discussions about the nature of the secondary qualities. The result has been a scarcity of work on the “other” secondary qualities. In this paper, I take smells and place them front and center. I ask: What are smells? For many philosophers, the view that colors can be explained in purely physicalistic terms has seemed very appealing. In the case of smells, this kind of nonrelational view has seemed much less appealing. Philosophers have been drawn to versions of relationalism—the view that the nature of smells must be explained (at least in part) in terms of the effects they have on perceivers. In this paper, I consider a contemporary argument for this view. I argue that nonrelationalist views of smell have little to fear from this argument. It was the first time Grenouille had ever been in a perfumery, a place in which odors are not accessories but stand unabashedly at the center of interest…. He knew every single odor handled here and had often merged them in his innermost thoughts to create the most splendid perfumes. - Patrick Süskind, Perfume In philosophical discussions of the secondary qualities, color has taken center stage. -

The Role of Memory and Attention in Change Blindness
Modeling the role that short term memory stores and attention play in change blindness Maarten Duijndam, Albert Postma & Stefan van der Stigchel Abstract Humans tend to think that they see the entire world. However change blindness – the inability to detect salient changes from one view to a next – reveals a different story. Objects in front of people can change without it being noticed. Change blindness does not occur due to poor visibility of the changes, because they can be clearly seen once they are detected. The question addressed in this paper concerns why these salient changes can so easily be missed. The relation between iconic memory, visual short term memory and attention is investigated. How can human access iconic and visual short term memory stores and use this information to detect changes? It is explained why these cognitive functions must operate together in order to detect changes. A new model is presented that describes the role that attention, iconic memory and Visual Short-Term Memory (VSTM) play. In order to detect changes attended stimuli from iconic memory or a fragile part of VSTM are transferred to a robust form of VSTM. This is necessary for change detection, because representations in the robust part of VSTM are solid enough to detect changes before they are lost from memory. Keywords Change blindness, attention, iconic memory and visual short term memory Introduction When people see a scene, they are convinced that they have a conscious representation of the entire scene. In contrast to what these persons think, research shows that they are not always aware of everything they see (Rensink, 2000). -

Edit Blindness: the Relationship Between Attention and Global Change Blindness in Dynamic Scenes
Journal of Eye Movement Research 2(2):6, 1-17 Edit Blindness: The relationship between attention and global change blindness in dynamic scenes. Tim J. Smith University of Edinburgh John M. Henderson University of Edinburgh Although we experience the visual world as a continuous, richly detailed space we often fail to notice large and significant changes. Such change blindness has been demonstrated for local object changes and changes to the visual form of whole images, however it is assumed that total changes from one image to another would be easily detected. Film editing presents such total changes several times a minute yet we rarely seem to be aware of them, a phenomenon we refer to here as edit blindness. This phenomenon has never been empirically demonstrated even though film editors believe they have at their disposal techniques that induce edit blindness, the Continuity Editing Rules. In the present study we tested the relationship between Continuity Editing Rules and edit blindness by instructing participants to detect edits while watching excerpts from feature films. Eye movements were recorded during the task. The results indicate that edits constructed according to the Continuity Editing Rules result in greater edit blindness than edits not adhering to the rules. A quarter of edits joining two viewpoints of the same scene were undetected and this increased to a third when the edit coincided with a sudden onset of motion. Some cuts may be missed due to suppression of the cut transients by coinciding with eyeblinks or saccadic eye movements but the majority seem to be due to inattentional blindness as viewers attend to the depicted narrative. -

Change Blindness
Change Blindness Ronald A. Rensink University of British Columbia In L. Itti, G. Rees, and J.K. Tsotsos (eds). Neurobiology of Attention. (pp. 76-81). 2005. San Diego, CA: Elsevier. Change Blindness Abstract Large changes that occur in clear view of an observer can become difficult to notice if made during an eye movement, blink, or other such disturbance. This change blindness is consistent with the proposal that focused visual attention is necessary to see change, with a change becoming difficult to notice whenever conditions prevent attention from being automatically drawn to it. It is shown here how the phenomenon of change blindness can provide new results on the nature of visual attention, including estimates of its capacity and the extent to which it can bind visual properties into coherent descriptions. It is also shown how the resultant characterization of attention can in turn provide new insights into the role that it plays in the perception of scenes and events. Change Blindness I. INTRODUCTION As observers, we have a strong impression that our visual system produces a coherent and detailed description of the world in front of us; a description, moreover, that is always stable and complete. However, various studies have shown that our ability to perceive objects and events in our visual field is far more limited than subjective experience indicates. Among the more striking phenomena in this regard is change blindness, the inability to notice changes that occur in clear view of the observer, even when these changes are large and the observer knows they will occur. (For a general review, see e.g., Rensink, 2002). -

An EEG Study of Detection Without Localisation in Change Blindness
An EEG study of detection without localisation in change blindness Article Published Version Creative Commons: Attribution 4.0 (CC-BY) Open Access Scrivener, C. L., Malik, A., Marsh, J., Lindner, M. and Roesch, E. B. (2019) An EEG study of detection without localisation in change blindness. Experimental Brain Research, 237 (10). pp. 2535-2547. ISSN 0014-4819 doi: https://doi.org/10.1007/s00221-019-05602-2 Available at http://centaur.reading.ac.uk/84852/ It is advisable to refer to the publisher’s version if you intend to cite from the work. See Guidance on citing . To link to this article DOI: http://dx.doi.org/10.1007/s00221-019-05602-2 Publisher: Springer All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in the End User Agreement . www.reading.ac.uk/centaur CentAUR Central Archive at the University of Reading Reading’s research outputs online Experimental Brain Research https://doi.org/10.1007/s00221-019-05602-2 RESEARCH ARTICLE An EEG study of detection without localisation in change blindness Catriona L. Scrivener1 · Asad Malik1 · Jade Marsh1 · Michael Lindner1 · Etienne B. Roesch1 Received: 7 January 2019 / Accepted: 8 July 2019 © The Author(s) 2019 Abstract Previous studies of change blindness have suggested a distinction between detection and localisation of changes in a visual scene. Using a simple paradigm with an array of coloured squares, the present study aimed to further investigate diferences in event-related potentials (ERPs) between trials in which participants could detect the presence of a colour change but not identify the location of the change (sense trials), versus those where participants could both detect and localise the change (localise trials). -

Cognitive Psychology: Attention, Memory, and Multitasking
Cognitive Psychology: Attention, Memory, and Multitasking by: Savannah Lewis, Rana Mumtaz, Ro-Anna Thomas, Leana Gomes, Jekiya Geter, Chelsea Morales, Gabriela Rodriguez What is Cognitive Psychology? • Cognitive Psychology is the study of how people perceive, remember, think, speak, and solve problems. Brain Diagram • Part A is called the frontal lobe o The prefrontal area is towards the front of the frontal cortex • Part E is called the Basal Ganglia A B • The brain works together as a C E whole to control our actions, o so we look at certain parts D of the brain to understand the thought processes of both people and animals. Introduction:Continued Attention • The mind’s ability to focus on what is going on in its surroundings o Manipulation of Attention . Top-down attention - the brain actively ignores or doesn't focus on something . Bottom-up attention- the brain directly focuses on something z • Change blindness: When people fail to notice changes to objects and scenes in their environment • Inattentional blindness: Failure to perceive objects and notice a change in environment because of the brain's focus on one thing at a time Introduction: Continued What is Multi-Tasking? • The brain is never actually focusing on two tasks at the same time, instead it is switching focus back and forth between two tasks; therefore it is actually split. o Split attention can be detrimental to quality, memory, performance, and accuracy. Multitasking can lead to: • Dual-task interference: which takes place when two cognitive tasks are done at the same time. And/Or • Continuous partial attention: when focus is continuously split between two or more tasks.