List of Measures Under Consideration for December 21, 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -
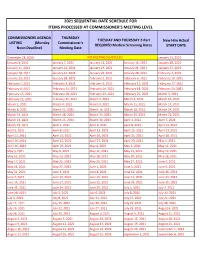
2021 Sequential Date List
2021 SEQUENTIAL DATE SCHEDULE FOR ITEMS PROCESSED AT COMMISSIONER'S MEETING LEVEL COMMISSIONERS AGENDA THURSDAY TUESDAY AND THURSDAY 2-Part New Hire Actual LISTING (Monday Commissioner's REQUIRED Medical Screening Dates START DATE Noon Deadline) Meeting Date December 28, 2020 NO MEETING SCHEDULED January 13, 2021 January 4, 2021 January 7, 2021 January 12, 2021 January 14, 2021 January 20, 2021 January 11, 2021 January 14, 2021 January 19, 2021 January 21, 2021 January 27, 2021 January 18, 2021 January 21, 2021 January 26, 2021 January 28, 2021 February 3, 2021 January 25, 2021 January 28, 2021 February 2, 2021 February 4, 2021 February 10, 2021 February 1, 2021 February 4, 2021 February 9, 2021 February 11, 2021 February 17, 2021 February 8, 2021 February 11, 2021 February 16, 2021 February 18, 2021 February 24, 2021 February 15, 2021 February 18, 2021 February 23, 2021 February 25, 2021 March 3, 2021 February 22, 2021 February 25, 2021 March 2, 2021 March 4, 2021 March 10, 2021 March 1, 2021 March 4, 2021 March 9, 2021 March 11, 2021 March 17, 2021 March 8, 2021 March 11, 2021 March 16, 2021 March 18, 2021 March 24, 2021 March 15, 2021 March 18, 2021 March 23, 2021 March 25, 2021 March 31, 2021 March 22, 2021 March 25, 2021 March 30, 2021 April 1, 2021 April 7, 2021 March 29, 2021 April 1, 2021 April 6, 2021 April 8, 2021 April 14, 2021 April 5, 2021 April 8, 2021 April 13, 2021 April 15, 2021 April 21, 2021 April 12, 2021 April 15, 2021 April 20, 2021 April 22, 2021 April 28, 2021 April 19, 2021 April 22, 2021 April 27, 2021 April -

Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January -

Great Conjunction” of Jupiter and Saturn
Astronomy Club of Asheville - December 2020 Highlight Page 1 of 3 The December 21, 2020 “Great Conjunction” of Jupiter and Saturn During the northern hemisphere summer of 2020, Jupiter and Saturn will become visible in our evening skies. Earth will approach closest to both these gas-giant planets in July – something astronomers call planetary opposition. By autumn, the 2 planets will appear to be on a slow collision course with each other in the constellation Sagittarius. But it’s in December that this very close alignment of Jupiter and Saturn reaches its culmination. The “great conjunction” of Jupiter and Saturn will occur on December 21, 2020 – the northern hemisphere’s winter solstice. At that time, the two planets will be in the constellation Capricornus, low toward the southwest horizon, and separated by a mere 0.1°. This will be the closest Jupiter/Saturn conjunction since the year 1623 CE! Jupiter will be at magnitude -2.0, and significantly dimmer Saturn at magnitude +0.6. On the evenings of December 16 & 17, 2020, the waxing crescent Long Night moon will join Jupiter and Saturn, making an amazing sight in the southwestern twilight. Above: Artist’s concept of Jupiter and Saturn on December 16, 2020 shortly after sunset, as viewed from Earth’s surface. Notice that a 7% illuminated, waxing crescent moon will also be part of this early evening view. Jupiter and Saturn will be rather low in the SW sky – only about 17° above the horizon in Asheville, NC. Chart via Jay Ryan at http://classicalastronomy.com/. - Continued on the next page - Astronomy Club of Asheville - December 2020 Highlight Page 2 of 3 The December 21, 2020 “Great Conjunction” of Jupiter and Saturn A “great conjunction” is a conjunction of the planets Jupiter and Saturn. -

Due Date Chart 201803281304173331.Xlsx
Special Event Permit Application Due Date Chart for Events from January 1, 2019 - June 30, 2020 If due date lands on a Saturday or Sunday, the due date is moved to the next business day Event Date 30 Calendar days 90 Calendar Days Tuesday, January 01, 2019 Sunday, December 02, 2018 Wednesday, October 03, 2018 Wednesday, January 02, 2019 Monday, December 03, 2018 Thursday, October 04, 2018 Thursday, January 03, 2019 Tuesday, December 04, 2018 Friday, October 05, 2018 Friday, January 04, 2019 Wednesday, December 05, 2018 Saturday, October 06, 2018 Saturday, January 05, 2019 Thursday, December 06, 2018 Sunday, October 07, 2018 Sunday, January 06, 2019 Friday, December 07, 2018 Monday, October 08, 2018 Monday, January 07, 2019 Saturday, December 08, 2018 Tuesday, October 09, 2018 Tuesday, January 08, 2019 Sunday, December 09, 2018 Wednesday, October 10, 2018 Wednesday, January 09, 2019 Monday, December 10, 2018 Thursday, October 11, 2018 Thursday, January 10, 2019 Tuesday, December 11, 2018 Friday, October 12, 2018 Friday, January 11, 2019 Wednesday, December 12, 2018 Saturday, October 13, 2018 Saturday, January 12, 2019 Thursday, December 13, 2018 Sunday, October 14, 2018 Sunday, January 13, 2019 Friday, December 14, 2018 Monday, October 15, 2018 Monday, January 14, 2019 Saturday, December 15, 2018 Tuesday, October 16, 2018 2019 Tuesday, January 15, 2019 Sunday, December 16, 2018 Wednesday, October 17, 2018 Wednesday, January 16, 2019 Monday, December 17, 2018 Thursday, October 18, 2018 Thursday, January 17, 2019 Tuesday, December 18, 2018 -

Seasons, Phases & Eclipses
The Seasons Autumn Winter Summer Sun Spring Winter Spring Summer Autumn Celestial Celestial Celestial Celestial Equator Equator Equator Equator Winter Observations What is the orientation of the earth’s axis in relation to the sun when the northern hemisphere experiences winter? (Pointed away) What is the sun’s position in relation to the celestial equator during the winter? (23 degrees below) Where on the globe (in latitude) would the sun appear directly overhead on the winter solstice? (Tropic of Capricorn, or 23 degrees south of the equator) 1 Summer Observations What is the orientation of the earth’s axis in relation to the sun when the northern hemisphere experiences summer? (Pointed towards) What is the sun’s position in relation to the celestial equator during the summer? (23 degrees above) Where on the globe (in latitude) would the sun appear directly overhead on the summer solstice? (Tropic of Cancer, or 23 degrees north of the equator) Comparison of Winter & Summer Compare the area of illumination for the winter and summer solstices. Which season experiences a greater intensity (greater concentration) of light? Summer Run the simulation again without stopping and note the distance and the speed of the earth as it orbits the sun. While the simulation is not to scale and greatly exaggerates the motion of the earth, it accurately depicts the changes in distance and speed of the earth as it orbits the sun. Comparison of speeds between summer and winter: The earth orbits the earth faster in the winter than the summer. Comparison of distances between summer and winter: The earth is closer to the sun in the winter than in the summer. -

“The Story of the Seasons”
“The Story of the Seasons” The Earth takes 365 and 1/4 days to complete one revolution around the sun and this amount of time is called a “year.” Every four years, 1/4 of a day will add up to 24- hour day, and we add an extra day (February 29th) to the calendar. This is why we have a “leap year” with one extra day every four years. The Earth’s orbit is nearly circular (or slightly elliptical) and Earth is actually closer to the sun during the northern hemisphere’s winter months. Summer On the first day of summer, June 20 or 21st, the Earth’s Northern Hemisphere is tilted 23.5° toward the sun. The day is known as the summer solstice. On this day the sun is at its highest point in the Northern Hemisphere sky at noon and directly over the Tropic of Cancer (the 23.5°N parallel of latitude). Solstice means “sun stop” in Latin. When the Northern Hemisphere is tilted toward the sun, that part of the Earth receives more direct rays of sunlight during the daytime than the Southern Hemisphere does. The Southern Hemisphere is tilted away from the sun and therefore, receives the sun’s rays at an angle. As a result, it is summer in the Northern Hemisphere and winter in the Southern Hemisphere. Conversely, during our winter months when the Northern Hemisphere is tilted away from the sun, it is summer in the Southern Hemisphere. During the summer, the land, oceans, and atmosphere in the Northern Hemisphere receive more direct rays of sunlight. -

Date of Close Contact Exposure
Date of Close Contact Exposure 7 days 10 days 14 days Monday, November 16, 2020 Tuesday, November 24, 2020 Friday, November 27, 2020 Tuesday, December 1, 2020 Tuesday, November 17, 2020 Wednesday, November 25, 2020 Saturday, November 28, 2020 Wednesday, December 2, 2020 Wednesday, November 18, 2020 Thursday, November 26, 2020 Sunday, November 29, 2020 Thursday, December 3, 2020 Thursday, November 19, 2020 Friday, November 27, 2020 Monday, November 30, 2020 Friday, December 4, 2020 Friday, November 20, 2020 Saturday, November 28, 2020 Tuesday, December 1, 2020 Saturday, December 5, 2020 Saturday, November 21, 2020 Sunday, November 29, 2020 Wednesday, December 2, 2020 Sunday, December 6, 2020 Sunday, November 22, 2020 Monday, November 30, 2020 Thursday, December 3, 2020 Monday, December 7, 2020 Monday, November 23, 2020 Tuesday, December 1, 2020 Friday, December 4, 2020 Tuesday, December 8, 2020 Tuesday, November 24, 2020 Wednesday, December 2, 2020 Saturday, December 5, 2020 Wednesday, December 9, 2020 Wednesday, November 25, 2020 Thursday, December 3, 2020 Sunday, December 6, 2020 Thursday, December 10, 2020 Thursday, November 26, 2020 Friday, December 4, 2020 Monday, December 7, 2020 Friday, December 11, 2020 Friday, November 27, 2020 Saturday, December 5, 2020 Tuesday, December 8, 2020 Saturday, December 12, 2020 Saturday, November 28, 2020 Sunday, December 6, 2020 Wednesday, December 9, 2020 Sunday, December 13, 2020 Sunday, November 29, 2020 Monday, December 7, 2020 Thursday, December 10, 2020 Monday, December 14, 2020 Monday, November -

Winter Solstice December 21, 2020 3:02 AM
Weather Forecast Office Winter Solstice Albuquerque, NM 2020 Updated: January 24, 2021 3:28 PM MDT December 21, 2020 3:02 AM MST The winter solstice occurs at the moment the earth's tilt away from the sun is at a maximum. Therefore, on the day of the winter solstice, the sun appears at its lowest elevation with a noontime position that changes very little for several days before and after the solstice. In fact, the word solstice comes from Latin solstitium or sol (the sun) + -stit-, -stes (standing). The winter solstice marks the shortest day and longest night of the year. In the Northern Hemisphere, it occurs when the sun is directly over the Tropic of Capricorn, which is located at 23.5° south of the equator and runs through Australia, Chile, southern Brazil, and northern South Africa. The Northern Hemisphere winter solstice will occur at 3:02 am MST on December 21, 2020. nasa.gov NWS Albuquerque weather.gov/abq Weather Forecast Office Winter Solstice Albuquerque, NM The Seasons Updated: January 24, 2021 3:28 PM MDT We all know that the Earth makes a complete revolution nasa.gov around the sun once every 365 days, following an orbit that is elliptical in shape. This means that the distance between the Earth and Sun, which is 93 million miles on average, varies throughout the year. The top of figure on the right illustrates that during the first week in January, the Earth is about 1.6 million miles closer to the sun. This is referred to as the perihelion. -

Bloomington Public Schools District 87 School Calendar 2021 - 2022
Bloomington Public Schools District 87 School Calendar 2021 - 2022 August ‘21 September ‘21 October ‘21 S M T W T F S S M T W T F S S M T W T F S 1 2 3 4 5 6 7 1 2 3 4 1 2 8 9 10 11 12 13 14 5 V 7 8 9 10 11 3 4 5 6 7 PTC 9 10 N 12 13 14 ¤ 16 15 ♦ ♦ ♦ (19 20 21 12 13 14 15 16 17 18 17 18 19 20 21 22 23 22 23 24 25 26 27 28 20 19 21 22 23 24 25 24 25 26 27 28 29 30 29 30 31 26 ♦ 28 29 30 31 November ‘21 December ‘21 January ‘22 S M T W T F S S M T W T F S S M T W T F S 1 1 2 3 4 5 6 1 2 3 4 2 S 4 5 6 7 8 7 8 9 10 11 12 13 5 6 7 8 9 10 11 9 10 11 12 13 14 15 12 13 14 15 16 ¤ 18 14 15 16 17 18 ø 20 16 V 18 19 20 21 22 21 22 23 N V N 27 19 N N N N N 25 23 24 25 26 27 28 29 28 29 30 26 N N N N N 30 31 February ‘22 March ‘22 April ‘22 S M T W T F S S M T W T F S S M T W T F S 1 2 3 4 5 1 2 3 4 5 1 2 6 7 8 9 10 11 12 6 7 8 9 10 ¤ 12 3 4 5 6 7 8 9 13 14 15 16 17 PTC 19 13 14 15 16 17 ½ ♦ 19 10 11 12 13 14 N 16 20 V 22 23 24 ø 26 20 N N N N N 26 17 18 19 20 21 22 23 27 28 27 28 29 30 31 24 25 26 27 28 29 30 Symbol Code May ‘22 June ‘22 Institute Days* ♦ First Day of School ( S M T W T F S S M T W T F S Holidays* V End of Quarterly Grading Period ¤ 1 2 3 4 5 6 7 E E E 4 End of Raymond Trimester ø Parent Teacher Conference* PTC 8 9 10 11 12 13 14 5 6 7 8 9 10 11 School Improvement Days* S 15 16 17 18 19 20 21 12 13 14 15 16 17 18 1/2 Day of Attendance ½ Last Day of School ) 22 23 24 )¤ø S E 28 19 20 21 22 23 24 25 Emergency Days E 29 V E Non Attendance Day* N 26 27 28 29 30 * = No Student Attendance Bloomington Public Schools District 87 Phone: 309/827-6031 Fax: 309/827-5717 300 E.