Emerging Trends for H1 Variant-Specific Functions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ncomms2254.Pdf
ARTICLE Received 3 May 2012 | Accepted 5 Nov 2012 | Published 4 Dec 2012 DOI: 10.1038/ncomms2254 The RB family is required for the self-renewal and survival of human embryonic stem cells Jamie F. Conklin1,2,3, Julie Baker2,3 & Julien Sage1,2,3 The mechanisms ensuring the long-term self-renewal of human embryonic stem cells are still only partly understood, limiting their use in cellular therapies. Here we found that increased activity of the RB cell cycle inhibitor in human embryonic stem cells induces cell cycle arrest, differentiation and cell death. Conversely, inactivation of the entire RB family (RB, p107 and p130) in human embryonic stem cells triggers G2/M arrest and cell death through functional activation of the p53 pathway and the cell cycle inhibitor p21. Differences in E2F target gene activation upon loss of RB family function between human embryonic stem cells, mouse embryonic stem cells and human fibroblasts underscore key differences in the cell cycle regulatory networks of human embryonic stem cells. Finally, loss of RB family function pro- motes genomic instability in both human and mouse embryonic stem cells, uncoupling cell cycle defects from chromosomal instability. These experiments indicate that a homeostatic level of RB activity is essential for the self-renewal and the survival of human embryonic stem cells. 1 Department of Pediatrics, Stanford Medical School, Stanford, California 94305, USA. 2 Department of Genetics, Stanford Medical School, Stanford, California 94305, USA. 3 Institute for Stem Cell Biology and Regenerative Medicine, Stanford Medical School, Stanford, California 94305, USA. Correspondence and requests for materials should be addressed to J.S. -

How Human H1 Histone Recognizes DNA
molecules Article How Human H1 Histone Recognizes DNA Olesya P. Luzhetskaya, Sergey E. Sedykh and Georgy A. Nevinsky * Institute of Chemical Biology and Fundamental Medicine, SD of Russian Academy of Sciences, 8 Lavrentiev Ave., 630090 Novosibirsk, Russia; [email protected] (O.P.L.); [email protected] (S.E.S.) * Correspondence: [email protected]; Tel.: +7-383-363-51-26; Fax: +7-383-363-51-53 Received: 11 August 2020; Accepted: 1 October 2020; Published: 5 October 2020 Abstract: Linker H1 histone is one of the five main histone proteins (H1, H2A, H2B, H3, and H4), which are components of chromatin in eukaryotic cells. Here we have analyzed the patterns of DNA recognition by free H1 histone using a stepwise increase of the ligand complexity method; the affinity of H1 histone for various single- and double-stranded oligonucleotides (d(pN)n; n = 1–20) was evaluated using their competition with 12-mer [32P]labeled oligonucleotide and protein–oligonucleotide complex delaying on nitrocellulose membrane filters. It was shown that minimal ligands of H1 histone (like other DNA-dependent proteins and enzymes) are different mononucleotides (dNMPs; Kd = (1.30 0.2) 2 ± 10 M). An increase in the length of single-stranded (ss) homo- and hetero-oligonucleotides (d(pA)n, × − d(pT)n, d(pC)n, and d(pN)n with different bases) by one nucleotide link regardless of their bases, leads to a monotonic increase in their affinity by a factor of f = 3.0 0.2. This factor f corresponds ± to the Kd value = 1/f characterizing the affinity of one nucleotide of different ss d(pN)n for H1 at n = 2–6 (which are covered by this protein globule) is approximately 0.33 0.02 M. -

Prospective Isolation of NKX2-1–Expressing Human Lung Progenitors Derived from Pluripotent Stem Cells
The Journal of Clinical Investigation RESEARCH ARTICLE Prospective isolation of NKX2-1–expressing human lung progenitors derived from pluripotent stem cells Finn Hawkins,1,2 Philipp Kramer,3 Anjali Jacob,1,2 Ian Driver,4 Dylan C. Thomas,1 Katherine B. McCauley,1,2 Nicholas Skvir,1 Ana M. Crane,3 Anita A. Kurmann,1,5 Anthony N. Hollenberg,5 Sinead Nguyen,1 Brandon G. Wong,6 Ahmad S. Khalil,6,7 Sarah X.L. Huang,3,8 Susan Guttentag,9 Jason R. Rock,4 John M. Shannon,10 Brian R. Davis,3 and Darrell N. Kotton1,2 2 1Center for Regenerative Medicine, and The Pulmonary Center and Department of Medicine, Boston University School of Medicine, Boston, Massachusetts, USA. 3Center for Stem Cell and Regenerative Medicine, Brown Foundation Institute of Molecular Medicine, University of Texas Health Science Center, Houston, Texas, USA. 4Department of Anatomy, UCSF, San Francisco, California, USA. 5Division of Endocrinology, Diabetes and Metabolism, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts, USA. 6Department of Biomedical Engineering and Biological Design Center, Boston University, Boston, Massachusetts, USA. 7Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, Massachusetts, USA. 8Columbia Center for Translational Immunology & Columbia Center for Human Development, Columbia University Medical Center, New York, New York, USA. 9Department of Pediatrics, Monroe Carell Jr. Children’s Hospital, Vanderbilt University, Nashville, Tennessee, USA. 10Division of Pulmonary Biology, Cincinnati Children’s Hospital, Cincinnati, Ohio, USA. It has been postulated that during human fetal development, all cells of the lung epithelium derive from embryonic, endodermal, NK2 homeobox 1–expressing (NKX2-1+) precursor cells. -
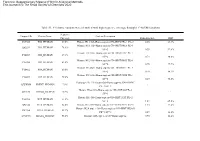
Table S1. 49 Histone Variants Were Identified with High Sequence Coverage Through LC-MS/MS Analysis Electronic Supplementary
Electronic Supplementary Material (ESI) for Analytical Methods. This journal is © The Royal Society of Chemistry 2020 Table S1. 49 histone variants were identified with high sequence coverage through LC-MS/MS analysis Sequence Uniprot IDs Protein Name Protein Description Coverage Ratio E2+/E2- RSD P07305 H10_HUMAN 67.5% Histone H1.0 OS=Homo sapiens GN=H1F0 PE=1 SV=3 4.85 23.3% Histone H1.1 OS=Homo sapiens GN=HIST1H1A PE=1 Q02539 H11_HUMAN 74.4% SV=3 0.35 92.6% Histone H1.2 OS=Homo sapiens GN=HIST1H1C PE=1 P16403 H12_HUMAN 67.1% SV=2 0.73 80.6% Histone H1.3 OS=Homo sapiens GN=HIST1H1D PE=1 P16402 H13_HUMAN 63.8% SV=2 0.75 77.7% Histone H1.4 OS=Homo sapiens GN=HIST1H1E PE=1 P10412 H14_HUMAN 69.0% SV=2 0.70 80.3% Histone H1.5 OS=Homo sapiens GN=HIST1H1B PE=1 P16401 H15_HUMAN 79.6% SV=3 0.29 98.3% Testis-specific H1 histone OS=Homo sapiens GN=H1FNT Q75WM6 H1FNT_HUMAN 7.8% \ \ PE=2 SV=3 Histone H1oo OS=Homo sapiens GN=H1FOO PE=2 Q8IZA3 H1FOO_HUMAN 5.2% \ \ SV=1 Histone H1t OS=Homo sapiens GN=HIST1H1T PE=2 P22492 H1T_HUMAN 31.4% SV=4 1.42 65.0% Q92522 H1X_HUMAN 82.6% Histone H1x OS=Homo sapiens GN=H1FX PE=1 SV=1 1.15 33.2% Histone H2A type 1 OS=Homo sapiens GN=HIST1H2AG P0C0S8 H2A1_HUMAN 99.2% PE=1 SV=2 0.57 26.8% Q96QV6 H2A1A_HUMAN 58.0% Histone H2A type 1-A OS=Homo sapiens 0.90 11.2% GN=HIST1H2AA PE=1 SV=3 Histone H2A type 1-B/E OS=Homo sapiens P04908 H2A1B_HUMAN 99.2% GN=HIST1H2AB PE=1 SV=2 0.92 30.2% Histone H2A type 1-C OS=Homo sapiens Q93077 H2A1C_HUMAN 100.0% GN=HIST1H2AC PE=1 SV=3 0.76 27.6% Histone H2A type 1-D OS=Homo sapiens P20671 -

UNIVERSITY of CALIFORNIA, SAN DIEGO Functional Analysis of Sall4
UNIVERSITY OF CALIFORNIA, SAN DIEGO Functional analysis of Sall4 in modulating embryonic stem cell fate A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Molecular Pathology by Pei Jen A. Lee Committee in charge: Professor Steven Briggs, Chair Professor Geoff Rosenfeld, Co-Chair Professor Alexander Hoffmann Professor Randall Johnson Professor Mark Mercola 2009 Copyright Pei Jen A. Lee, 2009 All rights reserved. The dissertation of Pei Jen A. Lee is approved, and it is acceptable in quality and form for publication on microfilm and electronically: ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ Co-Chair ______________________________________________________________ Chair University of California, San Diego 2009 iii Dedicated to my parents, my brother ,and my husband for their love and support iv Table of Contents Signature Page……………………………………………………………………….…iii Dedication…...…………………………………………………………………………..iv Table of Contents……………………………………………………………………….v List of Figures…………………………………………………………………………...vi List of Tables………………………………………………….………………………...ix Curriculum vitae…………………………………………………………………………x Acknowledgement………………………………………………….……….……..…...xi Abstract………………………………………………………………..…………….....xiii Chapter 1 Introduction ..…………………………………………………………………………….1 Chapter 2 Materials and Methods……………………………………………………………..…12 -

Table S1 the Four Gene Sets Derived from Gene Expression Profiles of Escs and Differentiated Cells
Table S1 The four gene sets derived from gene expression profiles of ESCs and differentiated cells Uniform High Uniform Low ES Up ES Down EntrezID GeneSymbol EntrezID GeneSymbol EntrezID GeneSymbol EntrezID GeneSymbol 269261 Rpl12 11354 Abpa 68239 Krt42 15132 Hbb-bh1 67891 Rpl4 11537 Cfd 26380 Esrrb 15126 Hba-x 55949 Eef1b2 11698 Ambn 73703 Dppa2 15111 Hand2 18148 Npm1 11730 Ang3 67374 Jam2 65255 Asb4 67427 Rps20 11731 Ang2 22702 Zfp42 17292 Mesp1 15481 Hspa8 11807 Apoa2 58865 Tdh 19737 Rgs5 100041686 LOC100041686 11814 Apoc3 26388 Ifi202b 225518 Prdm6 11983 Atpif1 11945 Atp4b 11614 Nr0b1 20378 Frzb 19241 Tmsb4x 12007 Azgp1 76815 Calcoco2 12767 Cxcr4 20116 Rps8 12044 Bcl2a1a 219132 D14Ertd668e 103889 Hoxb2 20103 Rps5 12047 Bcl2a1d 381411 Gm1967 17701 Msx1 14694 Gnb2l1 12049 Bcl2l10 20899 Stra8 23796 Aplnr 19941 Rpl26 12096 Bglap1 78625 1700061G19Rik 12627 Cfc1 12070 Ngfrap1 12097 Bglap2 21816 Tgm1 12622 Cer1 19989 Rpl7 12267 C3ar1 67405 Nts 21385 Tbx2 19896 Rpl10a 12279 C9 435337 EG435337 56720 Tdo2 20044 Rps14 12391 Cav3 545913 Zscan4d 16869 Lhx1 19175 Psmb6 12409 Cbr2 244448 Triml1 22253 Unc5c 22627 Ywhae 12477 Ctla4 69134 2200001I15Rik 14174 Fgf3 19951 Rpl32 12523 Cd84 66065 Hsd17b14 16542 Kdr 66152 1110020P15Rik 12524 Cd86 81879 Tcfcp2l1 15122 Hba-a1 66489 Rpl35 12640 Cga 17907 Mylpf 15414 Hoxb6 15519 Hsp90aa1 12642 Ch25h 26424 Nr5a2 210530 Leprel1 66483 Rpl36al 12655 Chi3l3 83560 Tex14 12338 Capn6 27370 Rps26 12796 Camp 17450 Morc1 20671 Sox17 66576 Uqcrh 12869 Cox8b 79455 Pdcl2 20613 Snai1 22154 Tubb5 12959 Cryba4 231821 Centa1 17897 -

Histone H1 Is Essential for Mitotic Chromosome Architecture
Published Online: 20 June, 2005 | Supp Info: http://doi.org/10.1083/jcb.200503031 JCB: ARTICLE Downloaded from jcb.rupress.org on May 12, 2019 Histone H1 is essential for mitotic chromosome architecture and segregation in Xenopus laevis egg extracts Thomas J. Maresca, Benjamin S. Freedman, and Rebecca Heald Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, CA 94720 uring cell division, condensation and resolution of spindle. Although functional kinetochores assembled, chromosome arms and the assembly of a func- aligned, and exhibited poleward movement, long and D tional kinetochore at the centromere of each sister tangled chromosome arms could not be segregated in chromatid are essential steps for accurate segregation of anaphase. Histone H1 depletion did not significantly affect the genome by the mitotic spindle, yet the contribution of the recruitment of known structural or functional chromo- individual chromatin proteins to these processes is poorly somal components such as condensins or chromokinesins, understood. We have investigated the role of embryonic suggesting that the loss of H1 affects chromosome archi- linker histone H1 during mitosis in Xenopus laevis egg tecture directly. Thus, our results indicate that linker histone extracts. Immunodepletion of histone H1 caused the assem- H1 plays an important role in the structure and function of bly of aberrant elongated chromosomes that extended off vertebrate chromosomes in mitosis. the metaphase plate and outside the perimeter of the Introduction Correct transmission of the genome by the mitotic spindle complexes condensin and cohesin, which are thought to form during cell division requires dramatic changes in chromosome ring structures that generate chromosome super coiling or architecture. -

Expression of the Human PAC1 Receptor Leads to Dose- Dependent Hydrocephalus-Related Abnormalities in Mice
Expression of the human PAC1 receptor leads to dose- dependent hydrocephalus-related abnormalities in mice Bing Lang, … , Anthony J. Harmar, Sanbing Shen J Clin Invest. 2006;116(7):1924-1934. https://doi.org/10.1172/JCI27597. Research Article Neuroscience Hydrocephalus is a common and potentially devastating birth defect affecting the CNS, and its relationship with G protein–coupled receptors (GPCRs) is unknown. We have expressed 2, 4, or 6 copies of a GPCR — the human PAC1 receptor with a 130-kb transgene in the mouse nervous system in a pattern closely resembling that of the endogenous gene. Consistent with PAC1 actions, PKA and PKC activity were elevated in the brains of Tg mice. Remarkably, Tg mice developed dose-dependent hydrocephalus-like characteristics, including enlarged third and lateral ventricles and reduced cerebral cortex, corpus callosum, and subcommissural organ (SCO). Neuronal proliferation and apoptosis were implicated in hydrocephalus, and we observed significantly reduced neuronal proliferation and massively increased neuronal apoptosis in the developing cortex and SCO of Tg embryos, while neurite outgrowth and neuronal migration in vitro remain uncompromised. Ventricular ependymal cilia are crucial for directing cerebrospinal fluid flow, and ependyma of Tg mice exhibited disrupted cilia with increased phospho-CREB immunoreactivity. These data demonstrate that altered neuronal proliferation/apoptosis and disrupted ependymal cilia are the main factors contributing to hydrocephalus in PAC1-overexpressing mice. This is the first report to our knowledge demonstrating that misregulation of GPCRs can be involved in hydrocephalus-related neurodevelopmental disorders. Find the latest version: https://jci.me/27597/pdf Research article Related Commentary, page 1828 Expression of the human PAC1 receptor leads to dose-dependent hydrocephalus- related abnormalities in mice Bing Lang,1 Bing Song,1 Wendy Davidson,1 Alastair MacKenzie,1 Norman Smith,2 Colin D. -

Snf2h-Mediated Chromatin Organization and Histone H1 Dynamics Govern Cerebellar Morphogenesis and Neural Maturation
ARTICLE Received 12 Feb 2014 | Accepted 15 May 2014 | Published 20 Jun 2014 DOI: 10.1038/ncomms5181 OPEN Snf2h-mediated chromatin organization and histone H1 dynamics govern cerebellar morphogenesis and neural maturation Matı´as Alvarez-Saavedra1,2, Yves De Repentigny1, Pamela S. Lagali1, Edupuganti V.S. Raghu Ram3, Keqin Yan1, Emile Hashem1,2, Danton Ivanochko1,4, Michael S. Huh1, Doo Yang4,5, Alan J. Mears6, Matthew A.M. Todd1,4, Chelsea P. Corcoran1, Erin A. Bassett4, Nicholas J.A. Tokarew4, Juraj Kokavec7, Romit Majumder8, Ilya Ioshikhes4,5, Valerie A. Wallace4,6, Rashmi Kothary1,2, Eran Meshorer3, Tomas Stopka7, Arthur I. Skoultchi8 & David J. Picketts1,2,4 Chromatin compaction mediates progenitor to post-mitotic cell transitions and modulates gene expression programs, yet the mechanisms are poorly defined. Snf2h and Snf2l are ATP-dependent chromatin remodelling proteins that assemble, reposition and space nucleosomes, and are robustly expressed in the brain. Here we show that mice conditionally inactivated for Snf2h in neural progenitors have reduced levels of histone H1 and H2A variants that compromise chromatin fluidity and transcriptional programs within the developing cerebellum. Disorganized chromatin limits Purkinje and granule neuron progenitor expansion, resulting in abnormal post-natal foliation, while deregulated transcriptional programs contribute to altered neural maturation, motor dysfunction and death. However, mice survive to young adulthood, in part from Snf2l compensation that restores Engrailed-1 expression. Similarly, Purkinje-specific Snf2h ablation affects chromatin ultrastructure and dendritic arborization, but alters cognitive skills rather than motor control. Our studies reveal that Snf2h controls chromatin organization and histone H1 dynamics for the establishment of gene expression programs underlying cerebellar morphogenesis and neural maturation. -

Transcriptional Regulation by Histone Ubiquitination and Deubiquitination
Downloaded from genesdev.cshlp.org on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press PERSPECTIVE Transcriptional regulation by histone ubiquitination and deubiquitination Yi Zhang1 Department of Biochemistry and Biophysics, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, North Carolina 27599, USA Ubiquitin (Ub) is a 76-amino acid protein that is ubiqui- The fact that histone ubiquitination occurs in the largely tously distributed and highly conserved throughout eu- monoubiquitinated form and is not linked to degrada- karyotic organisms. Whereas the extreme C-terminal tion, in combination with the lack of information regard- four amino acids are in a random coil, its N-terminal 72 ing the responsible enzymes, prevented us from under- amino acids have a tightly folded globular structure (Vi- standing the functional significance of this modification. jay-Kumar et al. 1987; Fig. 1A). Since its discovery ∼28 Recent identification of the E2 and E3 proteins involved years ago (Goldknopf et al. 1975), a variety of cellular in H2B ubiquitination (Robzyk et al. 2000; Hwang et al. processes including protein degradation, stress response, 2003; Wood et al. 2003a) and the discovery of cross-talk cell-cycle regulation, protein trafficking, endocytosis sig- between histone methylation and ubiquitination (Dover naling, and transcriptional regulation have been linked et al. 2002; Sun and Allis 2002) have set the stage for to this molecule (Pickart 2001). Ubiquitylation is pro- functional analysis of histone ubiquitination. In a timely posed to serve as a signaling module, and the informa- paper published in the previous issue of Genes & Devel- tion transmitted by this tag may depend on the nature of opment, Shelley Berger and colleagues (Henry et al. -

A Cytoplasmic Histone H1-Like Protein 2883 Ice-Cold RFB Buffer (160 Mm Kcl, 40 Mm Nacl, 20 Mm Na2egta, According to the Manufacturer’S Instruction
Research Article 2881 A novel linker histone-like protein is associated with cytoplasmic filaments in Caenorhabditis elegans Monika A. Jedrusik1, Stefan Vogt2, Peter Claus3 and Ekkehard Schulze1,* 1Georg-August University of Göttingen, Third Department of Zoology – Developmental Biology, Humboldtallee 34A, 37073 Göttingen, Germany 2Georg-August University of Göttingen, Institute for X-ray Physics, Geiststraße 11, 37073 Göttingen, Germany 3Hannover Medical School, Neuroanatomy, Carl-Neuberg-Str.1, 30625 Hannover, Germany *Author for correspondence (e-mail: [email protected]) Accepted 1 May 2002 Journal of Cell Science 115, 2881-2891 (2002) © The Company of Biologists Ltd Summary The histone H1 complement of Caenorhabditis elegans structure therein, the tonofilaments. Additionally contains a single unusual protein, H1.X. Although H1.X H1.X::GFP is expressed in the cytoplasm of body and vulva possesses the globular domain and the canonical three- muscle cells, neurons, excretory cells and in the nucleoli of domain structure of linker histones, the amino acid embryonic blastomeres and adult gut cells. RNA composition of H1.X is distinctly different from interference with H1.X results in uncoordinated and egg conventional linker histones in both terminal domains. We laying defective animals, as well as in a longitudinally have characterized H1.X in C. elegans by antibody labeling, enlarged pharynx. These phenotypes indicate a green fluorescent protein fusion protein expression and cytoplasmic role of H1.X in muscle growth and muscle RNA interference. Unlike normal linker histones, H1.X is function. a cytoplasmic as well as a nuclear protein and is not associated with chromosomes. H1.X is most prominently expressed in the marginal cells of the pharynx and is Key words: Caenorhabditis elegans, Histone H1, Chromatin, associated with a peculiar cytoplasmic cytoskeletal Intermediate filaments, Linker histone Introduction essential aspects of higher multicellular life, such as cell Linker histones are highly abundant eukaryotic chromatin differentiation and development. -

Drosophila Ribosomal Proteins Are Associated with Linker Histone H1 and Suppress Gene Transcription
Downloaded from genesdev.cshlp.org on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press Drosophila ribosomal proteins are associated with linker histone H1 and suppress gene transcription Jian-Quan Ni,1,3 Lu-Ping Liu,1,3 Daniel Hess,1 Jens Rietdorf,1 and Fang-Lin Sun1,2,4 1Friedrich Miescher Institute for Biomedical Research, Basel CH-4058, Switzerland; 2Institute of Epigenetics and Cancer Research, School of Medicine, Tsinghua University, Beijing 100080, China The dynamics and function of ribosomal proteins in the cell nucleus remain enigmatic. Here we provide evidence that specific components of Drosophila melanogaster ribosomes copurify with linker histone H1. Using various experimental approaches, we demonstrate that this association of nuclear ribosomal proteins with histone H1 is specific, and that colocalization occurs on condensed chromatin in vivo. Chromatin immunoprecipitation analysis confirmed that specific ribosomal proteins are associated with chromatin in a histone H1-dependent manner. Overexpression of either histone H1 or ribosomal protein L22 in Drosophila cells resulted in global suppression of the same set of genes, while depletion of H1 and L22 caused up-regulation of tested genes, suggesting that H1 and ribosomal proteins are essential for transcriptional gene repression. Overall, this study provides evidence for a previously undefined link between ribosomal proteins and chromatin, and suggests a role for this association in transcriptional regulation in higher eukaryotes. [Keywords: Ribosomal protein; L22; histone H1; chromatin; transcription] Supplemental material is available at http://www.genesdev.org. Received September 25, 2005; revised version accepted May 8, 2006. Transcription and translation in eukaryotes are generally discrete nuclear sites was sensitive to inhibitors of both believed to take place within two spatially separated cel- transcription and translation, arguing that the two pro- lular compartments.