Norovirus Gastroenteritis: Epidemiology, Clinical Manifestations, Infection Control Issues
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Noroviruses: Q&A
University of California, Berkeley 2222 Bancroft Way Berkeley, CA 94720 Appointments 510/642-2000 Online Appointment www.uhs.berkeley.edu Noroviruses: Q&A What are noroviruses? Noroviruses are a group of viruses that cause the “stomach flu” or gastroenteritis (GAS-tro-enter-I-tis) in people. The term “norovirus” was recently approved as the official name for this group of viruses. Several other names have been used for noroviruses, including: • Norwalk-like viruses (NLVs) • caliciviruses (because they belong to the virus family Caliciviridae) • small round structured viruses. Viruses are very different from bacteria and parasites, some of which can cause illnesses similar to norovirus infection. Viruses are much smaller, are not affected by treatment with antibiotics, and cannot grow outside of a person’s body. What are the symptoms of illness caused by noroviruses? The symptoms of norovirus illness usually include nausea, vomiting, diarrhea, and some stomach cramping. Sometimes people additionally have a low-grade fever, chills, headache, muscle aches and a general sense of tiredness. The illness often begins suddenly, and the infected person may feel very sick. The illness is usually brief, with symptoms lasting only about one or two days. In general, children experience more vomiting than adults. Most people with norovirus illness have both of these symptoms. What is the name of the illness caused by noroviruses? Illness caused by norovirus infection has several names, including: • stomach flu – this “stomach flu” is not related to the flu (or influenza), which is a respiratory illness caused by influenza virus • viral gastroenteritis – the most common name for illness caused by norovirus. -

Antiviral Bioactive Compounds of Mushrooms and Their Antiviral Mechanisms: a Review
viruses Review Antiviral Bioactive Compounds of Mushrooms and Their Antiviral Mechanisms: A Review Dong Joo Seo 1 and Changsun Choi 2,* 1 Department of Food Science and Nutrition, College of Health and Welfare and Education, Gwangju University 277 Hyodeok-ro, Nam-gu, Gwangju 61743, Korea; [email protected] 2 Department of Food and Nutrition, School of Food Science and Technology, College of Biotechnology and Natural Resources, Chung-Ang University, 4726 Seodongdaero, Daeduck-myun, Anseong-si, Gyeonggi-do 17546, Korea * Correspondence: [email protected]; Tel.: +82-31-670-4589; Fax: +82-31-676-8741 Abstract: Mushrooms are used in their natural form as a food supplement and food additive. In addition, several bioactive compounds beneficial for human health have been derived from mushrooms. Among them, polysaccharides, carbohydrate-binding protein, peptides, proteins, enzymes, polyphenols, triterpenes, triterpenoids, and several other compounds exert antiviral activity against DNA and RNA viruses. Their antiviral targets were mostly virus entry, viral genome replication, viral proteins, and cellular proteins and influenced immune modulation, which was evaluated through pre-, simultaneous-, co-, and post-treatment in vitro and in vivo studies. In particular, they treated and relieved the viral diseases caused by herpes simplex virus, influenza virus, and human immunodeficiency virus (HIV). Some mushroom compounds that act against HIV, influenza A virus, and hepatitis C virus showed antiviral effects comparable to those of antiviral drugs. Therefore, bioactive compounds from mushrooms could be candidates for treating viral infections. Citation: Seo, D.J.; Choi, C. Antiviral Bioactive Compounds of Mushrooms Keywords: mushroom; bioactive compound; virus; infection; antiviral mechanism and Their Antiviral Mechanisms: A Review. -

First Report and Genetic Characterization of Bovine Torovirus
Shi et al. BMC Veterinary Research (2020) 16:272 https://doi.org/10.1186/s12917-020-02494-1 RESEARCH ARTICLE Open Access First report and genetic characterization of bovine torovirus in diarrhoeic calves in China Zhihai Shi1,2† , Wenjia Wang3† , Chaoxi Chen4, Xiaozhan Zhang3* , Jing Wang1, Zhaoxue Xu1,2 and Yali Lan1* Abstract Background: Coronaviruses are notorious pathogens that cause diarrheic and respiratory diseases in humans and animals. Although the epidemiology and pathogenicity of coronaviruses have gained substantial attention, little is known about bovine coronavirus in cattle, which possesses a close relationship with human coronavirus. Bovine torovirus (BToV) is a newly identified relevant pathogen associated with cattle diarrhoea and respiratory diseases, and its epidemiology in the Chinese cattle industry remains unknown. Results: In this study, a total of 461 diarrhoeic faecal samples were collected from 38 different farms in three intensive cattle farming regions and analysed. Our results demonstrated that BToV is present in China, with a low prevalence rate of 1.74% (8/461). The full-length spike genes were further cloned from eight clinical samples (five farms in Henan Province). Phylogenetic analysis showed that two different subclades of BToV strains are circulating in China. Meanwhile, the three BToV strains identified from dairy calves, 18,307, 2YY and 5YY, all contained the amino acid variants R614Q, I801T, N841S and Q885E. Conclusions: This is the first report to confirm the presence of BToV in beef and dairy calves in China with diarrhea, which extend our understanding of the epidemiology of BToVs worldwide. Keywords: Bovine torovirus, China, Calf diarrhoea, Beef, Dairy, Phylogenetic analysis Background viruses and parasites. -

Guide for Common Viral Diseases of Animals in Louisiana
Sampling and Testing Guide for Common Viral Diseases of Animals in Louisiana Please click on the species of interest: Cattle Deer and Small Ruminants The Louisiana Animal Swine Disease Diagnostic Horses Laboratory Dogs A service unit of the LSU School of Veterinary Medicine Adapted from Murphy, F.A., et al, Veterinary Virology, 3rd ed. Cats Academic Press, 1999. Compiled by Rob Poston Multi-species: Rabiesvirus DCN LADDL Guide for Common Viral Diseases v. B2 1 Cattle Please click on the principle system involvement Generalized viral diseases Respiratory viral diseases Enteric viral diseases Reproductive/neonatal viral diseases Viral infections affecting the skin Back to the Beginning DCN LADDL Guide for Common Viral Diseases v. B2 2 Deer and Small Ruminants Please click on the principle system involvement Generalized viral disease Respiratory viral disease Enteric viral diseases Reproductive/neonatal viral diseases Viral infections affecting the skin Back to the Beginning DCN LADDL Guide for Common Viral Diseases v. B2 3 Swine Please click on the principle system involvement Generalized viral diseases Respiratory viral diseases Enteric viral diseases Reproductive/neonatal viral diseases Viral infections affecting the skin Back to the Beginning DCN LADDL Guide for Common Viral Diseases v. B2 4 Horses Please click on the principle system involvement Generalized viral diseases Neurological viral diseases Respiratory viral diseases Enteric viral diseases Abortifacient/neonatal viral diseases Viral infections affecting the skin Back to the Beginning DCN LADDL Guide for Common Viral Diseases v. B2 5 Dogs Please click on the principle system involvement Generalized viral diseases Respiratory viral diseases Enteric viral diseases Reproductive/neonatal viral diseases Back to the Beginning DCN LADDL Guide for Common Viral Diseases v. -
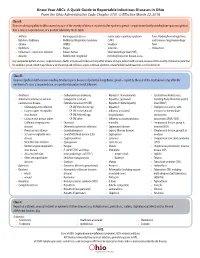
The Know Your Abcs: a Quick Guide to Reportable Infectious Diseases
Know Your ABCs: A Quick Guide to Reportable Infectious Diseases in Ohio From the Ohio Administrative Code Chapter 3701-3; Effective March 22, 2018 Class A: Diseases of major public health concern because of the severity of disease or potential for epidemic spread – report immediately via telephone upon recognition that a case, a suspected case, or a positive laboratory result exists. • Anthrax • Meningococcal disease • Severe acute respiratory syndrome fever, Marburg hemorrhagic fever, • Botulism, foodborne • Middle East Respiratory Syndrome (SARS) and Crimean-Congo hemorrhagic • Cholera (MERS) • Smallpox fever • Diphtheria • Plague • Tularemia • Yellow fever • Influenza A – novel virus infection • Rabies, human • Viral hemorrhagic fever (VHF), • Measles • Rubella (not congenital) including Ebola virus disease, Lassa Any unexpected pattern of cases, suspected cases, deaths or increased incidence of any other disease of major public health concern, because of the severity of disease or potential for epidemic spread, which may indicate a newly recognized infectious agent, outbreak, epidemic, related public health hazard or act of bioterrorism. Class B: Disease of public health concern needing timely response because of potential for epidemic spread – report by the end of the next business day after the existence of a case, a suspected case, or a positive laboratory result is known. • Amebiasis • Carbapenemase-producing • Hepatitis C (non-perinatal) • Spotted Fever Rickettsiosis, • Arboviral neuroinvasive and non- carbapenem-resistant • Hepatitis C (perinatal) including Rocky Mountain spotted neuroinvasive disease: Enterobacteriaceae (CP-CRE) • Hepatitis D (delta hepatitis) fever (RMSF) • Chikungunya virus infection • CP-CRE Enterobacter spp. • Hepatitis E • Staphylococcus aureus, with • Eastern equine encephalitis • CP-CRE Escherichia coli • Influenza-associated resistance or intermediate virus disease • CP-CRE Klebsiella spp. -

Reportable Diseases and Conditions
KINGS COUNTY DEPARTMENT of PUBLIC HEALTH 330 CAMPUS DRIVE, HANFORD, CA 93230 REPORTABLE DISEASES AND CONDITIONS Title 17, California Code of Regulations, §2500, requires that known or suspected cases of any of the diseases or conditions listed below are to be reported to the local health jurisdiction within the specified time frame: REPORT IMMEDIATELY BY PHONE During Business Hours: (559) 852-2579 After Hours: (559) 852-2720 for Immediate Reportable Disease and Conditions Anthrax Escherichia coli: Shiga Toxin producing (STEC), Rabies (Specify Human or Animal) Botulism (Specify Infant, Foodborne, Wound, Other) including E. coli O157:H7 Scrombroid Fish Poisoning Brucellosis, Human Flavivirus Infection of Undetermined Species Shiga Toxin (Detected in Feces) Cholera Foodborne Disease (2 or More Cases) Smallpox (Variola) Ciguatera Fish Poisoning Hemolytic Uremic Syndrome Tularemia, human Dengue Virus Infection Influenza, Novel Strains, Human Viral Hemorrhagic Fever (Crimean-Congo, Ebola, Diphtheria Measles (Rubeola) Lassa, and Marburg Viruses) Domonic Acid Poisoning (Amnesic Shellfish Meningococcal Infections Yellow Fever Poisoning) Novel Virus Infection with Pandemic Potential Zika Virus Infection Paralytic Shellfish Poisoning Plague (Specify Human or Animal) Immediately report the occurrence of any unusual disease OR outbreaks of any disease. REPORT BY PHONE, FAX, MAIL WITHIN ONE (1) WORKING DAY Phone: (559) 852-2579 Fax: (559) 589-0482 Mail: 330 Campus Drive, Hanford 93230 Conditions may also be reported electronically via the California -

Studies on Toroviruses of Humans and Cattle
Studies on toroviruses of humans and cattle Lynn Marie Duckmanton A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy, Graduate Department of Molecular and Medical Genetics, University of Toronto Copyright by Lynn M. Duckmanton, 1999 National Library Bibliotheque nationale 1*1 of Canada du Canada Acquisitions and Acquisitions et Bibliographic Services services bibliographiques 395 Wellington Street 395, rue Wellington OttawaON KIA ON4 OttawaON KlAON4 Canada Canada The author has granted a non- L'auteur a accorde une licence non exclusive licence allowing the exclusive pennettant a la National Library of Canada to Bibliotheque nationale du Canada de reproduce, loan, distribute or sell reprodwe, preter, distribuer ou copies of this thesis in microform, vendre des copies de cette these sous paper or electronic formats. la foxme de microfiche/film, de reproduction sur papier ou sur format Bectronique. The author retains ownership of the L'auteur conserve la propriete du copyright in this thesis. Neither the droit d'auteur qui protege cette ththe. thesis nor substantial extracts fiorn it Ni la these ni des extraits substantiels may be printed or otherwise de celle-ci ne doivent &re imprimes reproduced without the author's ou autrement reproduits sans son permission. autorisation. Studies on toroviruses of humans and cattle, Degree of Doctor of Philosophy, 1999, Lynn Marie Duckmanton, Graduate Department of Molecular and Medical Genetics, University of Toronto. Abstract Human torovirus (HTV) was purified from patient stool specimens and characterized. By negative contrast electron microscopy (EM) and thin-section EM, torovirus-like particles were found to be morphologically similar to Berne virus (BEV) and Breda virus (BRV). -

Growth of Escherichia Coli O157:H7 and Salmonella Serovars on Raw Beef, Pork, Chicken, Bratwurst and Cured Corned Beef: Implications for Haccp Plan Critical Limits
Blackwell Science, LtdOxford, UKJFSJournal of Food Safety1526-2375by Food & Nutrition Press, Inc., Trumbull, Connecticut2004244246256Original Article GROWTH OF PATHOGENS ON RAW MEAT PRODUCTSS.C. INGHAM ET AL. GROWTH OF ESCHERICHIA COLI O157:H7 AND SALMONELLA SEROVARS ON RAW BEEF, PORK, CHICKEN, BRATWURST AND CURED CORNED BEEF: IMPLICATIONS FOR HACCP PLAN CRITICAL LIMITS STEVEN C. INGHAM1, JILL A. LOSINSKI and KATIE L. BECKER 1605 Linden Drive Department of Food Science University of Wisconsin-Madison Madison, Wisconsin 53706 AND DENNIS R. BUEGE 1805 Linden Drive West Department of Animal Sciences University of Wisconsin-Madison Madison, Wisconsin 53706 Accepted for Publication June 7, 2004 ABSTRACT Small amounts (10–25 g; 6.3–20.8 cm2 inoculated area) of raw ground beef, intact beef, pork and chicken (dark and white meat),and bratwurst and cured corned beef were inoculated with Salmonella serovars and Escherichia coli O157:H7, refrigerated 24 h at 5C, and then held either at 10C (± 1C) for up to 8 h or at room temperature (22C ± 2C) for up to 2 h. Except for a 0.2 log CFU increase in Salmonella serovars in ground beef during 2 h at room temperature, pathogens did not grow. Results of trials with commercial amounts of beef, pork, chicken, ground beef and bratwurst exposed to 10C for 8 h or 22C for 2 h also showed no pathogen growth. Potential critical limits for processing of previously refrigerated raw meat products are exposure temperatures between 5 and 10C for not more than 8 h or between 5 and 22C for not more than 2 h. 1 Author for correspondence. -

Norovirus Infectious Agent Information Sheet
Norovirus Infectious Agent Information Sheet Introduction Noroviruses are non-enveloped (naked) RNA viruses with icosahedral nucleocapsid symmetry. The norovirus genome consists of (+) ssRNA, containing three open reading frames that encode for proteins required for transcription, replication, and assembly. There are five norovirus genogroups (GI-GV), and only GI, GII, and GIV infect humans. Norovirus belongs to the Caliciviridae family of viruses, and has had past names including, Norwalk virus and “winter-vomiting” disease. Epidemiology and Clinical Significance Noroviruses are considered the most common cause of outbreaks of non-bacterial gastroenteritis worldwide, are the leading cause of foodborne illness in the United States (58%), and account for 26% of hospitalizations and 10% of deaths associated with food consumption. Salad ingredients, fruit, and oysters are the most implicated in norovirus outbreaks. Aside from food and water, Noroviruses can also be transmitted by person to person contact and contact with environmental surfaces. The rapid spread of secondary infections occurs in areas where a large population is enclosed within a static environment, such as cruise ships, military bases, and institutions. Symptoms typically last for 24 to 48 hours, but can persist up to 96 hours in the immunocompromised. Pathogenesis, Immunity, Treatment and Prevention Norovirus is highly infectious due to low infecting dose, high excretion level (105 to 107 copies/mg stool), and continual shedding after clinical recovery (>1 month). The norovirus genome undergoes frequent change due to mutation and recombination, which increases its prevalence. Studies suggest that acquired immunity only last 6 months after infection. Gastroenteritis, an inflammation of the stomach and small and large intestines, is caused by norovirus infection. -

Fact Sheet Norovirus
New Hampshire Department of Health and Human Services Fact Sheet Division of Public Health Services Norovirus What is norovirus? How is norovirus infection diagnosed? Noroviruses are a group of viruses that cause Laboratory diagnosis is difficult but there are the “stomach flu,” or gastrointestinal tests that can be performed in the New (stomach and digestive) illness. Norovirus Hampshire Public Health Lab in situations infection occurs occasionally in only one or a where there are multiple cases. Diagnosis is few people or it can be responsible for large often based on the combination of symptoms outbreaks, such as in long-term care facilities. and the short time of the illness. Who gets norovirus? What is the treatment for norovirus Norovirus infects people of all ages infection? worldwide. It may, however, be more No specific treatment is available. People who common in adults and older children. become dehydrated might need to be rehydrated by taking liquids by mouth. How does someone get norovirus? Occasionally patients may need to be Norovirus is spread from person to person via hospitalized to receive intravenous fluids. feces, but some evidence suggests that the virus is spread through the air during How can norovirus be prevented? vomiting. Good hand washing is the most While there is no vaccine for norovirus, there important way to prevent the transmission of are precautions people should take: norovirus. Outbreaks have been linked to sick • Wash hands with soap and warm water food handlers, ill health care workers, cases in after using the bathroom and after facilities such as nursing homes spreading to changing diapers other residents, contaminated shellfish, and • Wash hands with soap and warm water water contaminated with sewage. -

Metagenomic Analysis of the Turkey Gut RNA Virus Community J Michael Day1*, Linda L Ballard2, Mary V Duke2, Brian E Scheffler2, Laszlo Zsak1
Day et al. Virology Journal 2010, 7:313 http://www.virologyj.com/content/7/1/313 RESEARCH Open Access Metagenomic analysis of the turkey gut RNA virus community J Michael Day1*, Linda L Ballard2, Mary V Duke2, Brian E Scheffler2, Laszlo Zsak1 Abstract Viral enteric disease is an ongoing economic burden to poultry producers worldwide, and despite considerable research, no single virus has emerged as a likely causative agent and target for prevention and control efforts. Historically, electron microscopy has been used to identify suspect viruses, with many small, round viruses eluding classification based solely on morphology. National and regional surveys using molecular diagnostics have revealed that suspect viruses continuously circulate in United States poultry, with many viruses appearing concomitantly and in healthy birds. High-throughput nucleic acid pyrosequencing is a powerful diagnostic technology capable of determining the full genomic repertoire present in a complex environmental sample. We utilized the Roche/454 Life Sciences GS-FLX platform to compile an RNA virus metagenome from turkey flocks experiencing enteric dis- ease. This approach yielded numerous sequences homologous to viruses in the BLAST nr protein database, many of which have not been described in turkeys. Our analysis of this turkey gut RNA metagenome focuses in particular on the turkey-origin members of the Picornavirales, the Caliciviridae, and the turkey Picobirnaviruses. Introduction remains elusive, and many enteric viruses can be Enteric disease syndromes such as Poult Enteritis Com- detected in otherwise healthy turkey and chicken flocks plex (PEC) in young turkeys and Runting-Stunting Syn- [3,4]. Regional and national enteric virus surveys have drome (RSS) in chickens are a continual economic revealed the ongoing presence of avian reoviruses, rota- burden for poultry producers. -

Rotavirus Vaccine Contraindicated in Infants with Severe Combined Immunodeficiency
The National Patient Organization Dedicated to Advocacy, Education and Research for Primary Immunodeficiency Diseases From Medscape Medical News Rotavirus Vaccine Contraindicated in Infants With Severe Combined Immunodeficiency Brande Nicole Martin June 11, 2010—Rotavirus vaccine should not be administered to infants with severe combined immunodeficiency (SCID), according to the Centers for Disease Control and Prevention (CDC) and US Food and Drug Administration (FDA)-approved prescribing information and patient safety labeling. Both monovalent (RV1) and pentavalent (RV5) rotavirus vaccines are contraindicated in infants diagnosed with SCID and can cause vaccine-acquired infection. The CDC announced this new contraindication to rotavirus vaccine in the June 11 issue of Morbidity and Mortality Weekly Report. SCID is a rare, life-threatening group of disorders caused by defects in several genes that is commonly diagnosed in infants after they have experienced a severe, potentially life-threatening infection from one or more pathogens. It occurs annually in about 40 to 100 new cases in the United States per year. Most infants commonly experience chronic diarrhea, failure to thrive, and early onset of infections. In December 2009 and February 2010, Merck Co and GlaxoSmithKline Biologicals, the makers of the RV1 (RotaTeq) and RV5 (Rotarix) vaccines, respectively, updated their prescribing information and patient labeling with FDA approval to reflect this contraindication. The CDC also has updated their list of contraindications to rotavirus vaccine after consulting with the Advisory Committee on Immunization Practices. The addition of this contraindication was based on data from 8 reported cases of vaccine-acquired rotavirus infection in infants with SCID since 2006, when the rotavirus vaccine was first introduced in the United States.