Smith, Aimee (2018) Volatile Elements in Martian Apatite: Insights from New Meteorites NWA 7034 and NWA 8159
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
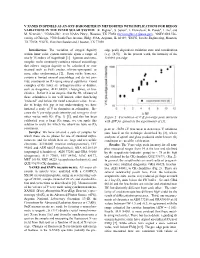
V Xanes in Spinels As an Oxy-Barometer in Meteorites with Implications for Redox Variations in the Inner Solar System
V XANES IN SPINELS AS AN OXY-BAROMETER IN METEORITES WITH IMPLICATIONS FOR REDOX VARIATIONS IN THE INNER SOLAR SYSTEM. K. Righter1, S. Sutton2, L. Danielson3, K. Pando4, L. Le3, and M. Newville2. 1NASA-JSC, 2101 NASA Pkwy., Houston, TX 77058 ([email protected]), 2GSECARS Uni- versity of Chicago, 9700 South Cass Avenue, Bldg. 434A, Argonne, IL 60439; 3ESCG, Jacobs Engineering, Houston, TX 77058; 4ESCG, Hamilton Sundstrand, Houston, TX 77058 Introduction: The variation of oxygen fugacity edge peak) depend on oxidation state and coordination within inner solar system materials spans a range of (e.g., [4,5]). In the present work, the intensity of the nearly 15 orders of magnitiude [1]. Igneous and meta- XANES pre-edge morphic rocks commonly contain a mineral assemblage that allows oxygen fugacity to be calculated or con- strained such as FeTi oxides, olivine-opx-spinel, or some other oxybarometer [2]. Some rocks, however, contain a limited mineral assemblage and do not pro- vide constraints on fO2 using mineral equilibria. Good examples of the latter are orthopyroxenites or dunites, such as diogenites, ALH 84001, chassignites, or bra- chinites. In fact it is no surprise that the fO2 of many of these achondrites is not well known, other than being "reduced" and below the metal saturation value. In or- der to bridge this gap in our understanding, we have initiated a study of V in chromites in achondrite. Be- cause the V pre-edge peak intensity and energy in chro- mites varies with fO2 (Fig. 1) [3], and this has been Figure 1: Correlation of V K pre-edge peak intensity calibrated over a large fO2 range, we can apply this with IW for spinels in the experiments of [3]. -

SPECTRAL CHARACTERIZATION of the ANCIENT SHERGOTTITES NORTHWEST AFRICA 7034 and 8159. KJ Orr1, LV Forman1, GK Benedix1
Ninth International Conference on Mars 2019 (LPI Contrib. No. 2089) 6177.pdf SPECTRAL CHARACTERIZATION OF THE ANCIENT SHERGOTTITES NORTHWEST AFRICA 7034 AND 8159. K. J. Orr1, L. V. Forman1, G. K. Benedix1, M. J. Hackett2, V. E. Hamilton3, and A. R. Santos. 1Space Science and Technology Centre (SSTC), School of Earth and Planetary Sciences, Curtin University, Perth, Western Australia, Australia ([email protected]), 2School of Molecular and Life Sciences, Curtin University, Perth, Western Australia, Australia, 3Southwest Research Institute, 1050 Walnut St. #300, Boulder, CO 80302 USA. 4USRA, 7178 Columbia Gateway Dr., Columbia, MD 21046. Introduction: Thermal infrared (TIR) spectros- Ga), the oldest confirmed shergottites recovered so far copy is a powerful remote sensing tool used to unravel [5]. As the only shergottites of Early Amazonian to No- the surface compositions of a target body. This tech- achian in age, they provide an invaluable opportunity to nique has been widely used in space missions, because understanding Mars’ early history. of its ability to detect and determine modal mineralogy Methods: Both samples (~0.5g chips) were ac- of the surface geology. It has been instrumental in de- quired from UNM and were made into epoxy mounts. veloping our understanding of Mars, as the majority of NWA 8159 was analyzed with a Tescan Integrated Min- missions sent to Mars have included an infrared spec- eral Analyzer (TIMA) to determine modal mineral trometer. These spectrometers can operate either in the abundancies and produce high-resolution mineral maps. visible (VIS) to near-infrared (NIR) or in the mid-infra- NWA 8159 was also analyzed using EBSD to charac- red (MIR). -

Physical Properties of Martian Meteorites: Porosity and Density Measurements
Meteoritics & Planetary Science 42, Nr 12, 2043–2054 (2007) Abstract available online at http://meteoritics.org Physical properties of Martian meteorites: Porosity and density measurements Ian M. COULSON1, 2*, Martin BEECH3, and Wenshuang NIE3 1Solid Earth Studies Laboratory (SESL), Department of Geology, University of Regina, Regina, Saskatchewan S4S 0A2, Canada 2Institut für Geowissenschaften, Universität Tübingen, 72074 Tübingen, Germany 3Campion College, University of Regina, Regina, Saskatchewan S4S 0A2, Canada *Corresponding author. E-mail: [email protected] (Received 11 September 2006; revision accepted 06 June 2007) Abstract–Martian meteorites are fragments of the Martian crust. These samples represent igneous rocks, much like basalt. As such, many laboratory techniques designed for the study of Earth materials have been applied to these meteorites. Despite numerous studies of Martian meteorites, little data exists on their basic structural characteristics, such as porosity or density, information that is important in interpreting their origin, shock modification, and cosmic ray exposure history. Analysis of these meteorites provides both insight into the various lithologies present as well as the impact history of the planet’s surface. We present new data relating to the physical characteristics of twelve Martian meteorites. Porosity was determined via a combination of scanning electron microscope (SEM) imagery/image analysis and helium pycnometry, coupled with a modified Archimedean method for bulk density measurements. Our results show a range in porosity and density values and that porosity tends to increase toward the edge of the sample. Preliminary interpretation of the data demonstrates good agreement between porosity measured at 100× and 300× magnification for the shergottite group, while others exhibit more variability. -

Magnetite Biomineralization and Ancient Life on Mars Richard B Frankel* and Peter R Buseckt
Magnetite biomineralization and ancient life on Mars Richard B Frankel* and Peter R Buseckt Certain chemical and mineral features of the Martian meteorite with a mass distribution unlike terrestrial PAHs or those from ALH84001 were reported in 1996 to be probable evidence of other meteorites; thirdly, bacterium-shaped objects (BSOs) ancient life on Mars. In spite of new observations and up to several hundred nanometers long that resemble fos interpretations, the question of ancient life on Mars remains silized terrestrial microorganisms; and lastly, 10-100 nm unresolved. Putative biogenic, nanometer magnetite has now magnetite (Fe304), pyrrhotite (Fel_xS), and greigite (Fe3S4) become a leading focus in the debate. crystals. These minerals were cited as evidence because of their similarity to biogenic magnetic minerals in terrestrial Addresses magnetotactic bacteria. *Department of Physics, California Polytechnic State University, San Luis Obispo, California 93407, USA; e-mail: [email protected] The ancient life on Mars hypothesis has been extensively tDepartments of Geology and Chemistry/Biochemistry, Arizona State challenged, and alternative non-biological processes have University, Tempe, Arizona 85287-1404, USA; e-mail: [email protected] been proposed for each of the four features cited by McKay et al. [4]. In this paper we review the current situa tion regarding their proposed evidence, focusing on the Abbreviations putative biogenic magnetite crystals. BCM biologically controlled mineralization BIM biologically induced mineralization BSO bacterium-shaped object Evidence for and against ancient Martian life PAH polycyclic aromatic hydrocarbon PAHs and BSOs Reports of contamination by terrestrial organic materials [5°,6°] and the similarity of ALH84001 PAHs to non-bio genic PAHs in carbonaceous chondrites [7,8] make it Introduction difficult to positively identify PAHs of non-terrestrial, bio A 2 kg carbonaceous stony meteorite, designated genic origin. -

Moore County Unbrecciated Cumulate Eucrite, 1.88Kg
Moore County Unbrecciated Cumulate Eucrite, 1.88 kg Seen to fall Figure 1a: The Moore County eucrite (fusion crust on left), from the collection of the North Carolina Museum of Natural Sciences. Scale from top to bottom of sample is ~ 5 cm. Photo courtesy of Chris Tacker. Introduction: The Moore County meteorite (Figures 1a,b,c) fell at 5:00 PM on April 21, 1913, on the farm of George C. Graves, located approximately three miles east of Carthage, Moore County, North Carolina (79o23’W, 35o25’N) (Henderson and Davis, 1936). A loud “rumbling and zooming” noise “with no distinct explosions” was first observed within a five or six mile radius of the fall, followed by a sighting of a red hot ball with a 15-foot trail of blue-black smoke; the meteorite itself landed within a few feet of a farmer, in a nearly-vertical (but slightly SW-sloping) hole in a freshly-plowed field (Henderson and Davis, 1936). Only one stone was recovered (Figure 1b), weighing approximately 1.88 kg (4 lbs. 2 oz.), with maximum dimensions approximately 15 cm x 10.5 cm x 8 cm (6 in x 4 3/16 in x 3 3/16 in) (Henderson and Davis, 1936). This stone was divided between the US National Museum (Smithsonian) in Washington, D.C., and the North Carolina State Museum in Raleigh, now the North Carolina Museum of Natural Sciences (Henderson and Davis, 1936), where the main fractions of the stone are still kept (0.9 kg at the USNM and ~0.56 kg at the NC Museum: Grady, 2000; Tacker, pers. -

The Investigated Rock Is One of the Gabbroic Bodies Which Crops out in the Surroundings of Lyngdal ( Extreme South of Norway)
PYROXENE RELATIONS IN A HYPERITE NEAR LYNGDAL, NORWAY JOHAN J. LAVREAU Lavreau, J. J.: Pyroxene relations in a hyperite near Lyngdal, Norway. Norsk Geologisk Tidsskrift, Vol. 50, pp. 333-340. Oslo 1970. Pyroxenes from a hyperite body associated with Precambrian gneisses were studied optically, chemically, and by means of X-rays. Pyroxene relations show that crystallization takes place at a temperature higher than the inversion of pigeonite, and proceeds into the stability field of hypersthene. The composition and the mutual relations between the pyroxenes suggest a crystallization process for these minerals. Johan J. Lavreau, Laboratoire de Mineralogie et Petrologie, Universite Libre de Bruxelles, Brussels, Belgium. Introduction The investigated rock is one of the gabbroic bodies which crops out in the surroundings of Lyngdal ( extreme South of Norway). It Iies 3 km north of the town, northwest of Skoland lake, and is crossed by the E 18 road to Flekkefjord (Fig. 1). It is a well defined and homogeneous crescent-shaped unit, about 1500 m long and 500 m broad. The rock is medium grained (1-2 mm grain size), dark grey in colour, and has a gabbro-dioritic compo sition (Niggli 1923, p. 126). Some dioritic varieties were also recognized (Table 1). The country rock is a monzonitic phenoblastic gneiss foliated parallel to the elongation of the hyperite body. The contacts are seldom visible, for the area is covered by recent deposits except in its northern part, where the rock is unfortunately tectonized and strongly retrometa morphosed. Experimental techniques Plagioclase and pyroxenes were first studied with the aid of the universal stage. -

March 21–25, 2016
FORTY-SEVENTH LUNAR AND PLANETARY SCIENCE CONFERENCE PROGRAM OF TECHNICAL SESSIONS MARCH 21–25, 2016 The Woodlands Waterway Marriott Hotel and Convention Center The Woodlands, Texas INSTITUTIONAL SUPPORT Universities Space Research Association Lunar and Planetary Institute National Aeronautics and Space Administration CONFERENCE CO-CHAIRS Stephen Mackwell, Lunar and Planetary Institute Eileen Stansbery, NASA Johnson Space Center PROGRAM COMMITTEE CHAIRS David Draper, NASA Johnson Space Center Walter Kiefer, Lunar and Planetary Institute PROGRAM COMMITTEE P. Doug Archer, NASA Johnson Space Center Nicolas LeCorvec, Lunar and Planetary Institute Katherine Bermingham, University of Maryland Yo Matsubara, Smithsonian Institute Janice Bishop, SETI and NASA Ames Research Center Francis McCubbin, NASA Johnson Space Center Jeremy Boyce, University of California, Los Angeles Andrew Needham, Carnegie Institution of Washington Lisa Danielson, NASA Johnson Space Center Lan-Anh Nguyen, NASA Johnson Space Center Deepak Dhingra, University of Idaho Paul Niles, NASA Johnson Space Center Stephen Elardo, Carnegie Institution of Washington Dorothy Oehler, NASA Johnson Space Center Marc Fries, NASA Johnson Space Center D. Alex Patthoff, Jet Propulsion Laboratory Cyrena Goodrich, Lunar and Planetary Institute Elizabeth Rampe, Aerodyne Industries, Jacobs JETS at John Gruener, NASA Johnson Space Center NASA Johnson Space Center Justin Hagerty, U.S. Geological Survey Carol Raymond, Jet Propulsion Laboratory Lindsay Hays, Jet Propulsion Laboratory Paul Schenk, -

Apatite from NWA 10153 and NWA 10645—The Key to Deciphering Magmatic and Fluid Evolution History in Nakhlites
minerals Article Apatite from NWA 10153 and NWA 10645—The Key to Deciphering Magmatic and Fluid Evolution History in Nakhlites Łukasz Birski 1,*, Ewa Słaby 1, Elias Chatzitheodoridis 2, Richard Wirth 3, Katarzyna Majzner 4, Gabriela A. Kozub-Budzy ´n 5, Jiˇrí Sláma 6, Katarzyna Liszewska 1, Izabela Kocjan 7 and Anna Zagórska 7 1 Institute of Geological Sciences, Polish Academy of Sciences, Research Centre in Warsaw, Twarda 51/55, 00-818 Warsaw, Poland; [email protected] (E.S.); [email protected] (K.L.) 2 Department of Geological Sciences, School of Mining and Metallurgical Engineering, National Technical University of Athens, 9 Heroon Polytechneiou, 15780 Zografou, Athens, Greece; [email protected] 3 Helmholtz-Zentrum Potsdam Deutsches GeoForschungsZentrum GFZ, Telegrafenberg, 14473 Potsdam, Germany; [email protected] 4 Faculty of Chemistry, Jagiellonian University, Raman Imaging Group, Gronostajowa 2, 30-387 Cracow, Poland; [email protected] 5 Faculty of Geology, Geophysics and Environmental Protection, AGH University of Science and Technology, Mickiewicza 30, 30-059 Cracow, Poland; [email protected] 6 Institute of Geology Academy of Sciences of the Czech Republic Rozvojová 269, 165 00 Prague Czech Republic; [email protected] 7 Institute of Geological Sciences, Polish Academy of Sciences, Research Centre in Cracow, Senacka 1, 31-002 Cracow, Poland; [email protected] (I.K.); [email protected] (A.Z.) * Correspondence: [email protected] Received: 10 September 2019; Accepted: 7 November 2019; Published: 10 November 2019 Abstract: Apatites from Martian nakhlites NWA 10153 and NWA 10645 were used to obtain insight into their crystallization environment and the subsequent postcrystallization evolution path. -

Constraints on the Depth and Thermal Vigor of Melting in the Martian Mantle
PUBLICATIONS Journal of Geophysical Research: Planets RESEARCH ARTICLE Constraints on the depth and thermal vigor 10.1002/2014JE004745 of melting in the Martian mantle Key Points: Justin Filiberto1 and Rajdeep Dasgupta2 • Mantle potential temperature calculated for Gale Crater rocks 1Department of Geology, Southern Illinois University, Carbondale, Illinois, USA, 2Department of Earth Science, Rice • 1450 ± 70°C may represent global Noachian mantle temperature University, Houston, Texas, USA • Consistent with simple convective cooling of the interior of Mars Abstract Studies of rocks in Gale Crater and clasts within the Martian meteorite breccia Northwest Africa (NWA) 7034 (and paired stones) have expanded our knowledge of the diversity of igneous rocks that make up the Martian crust beyond those compositions exhibited in the meteorite collection or Correspondence to: J. Filiberto, analyzed at any other landing site. Therefore, the magmas that gave rise to these rocks may have been fi[email protected] generated at significantly different conditions in the Martian mantle than those derived from previously studied rocks. Here we build upon our previous models of basalt formation based on rocks analyzed in Citation: Gusev Crater and Meridiani Planum to the new models of basalt formation for compositions from Gale Filiberto, J., and R. Dasgupta (2015), Crater and a clast in meteorite NWA 7034. Estimates for the mantle potential temperature, TP based on Constraints on the depth and thermal vigor of melting in the Martian mantle, Noachian age rock analyses in Gale Crater, Gusev Crater, and Bounce Rock in Meridiani Planum, and a J. Geophys. Res. Planets, 120, vitrophyre clast in NWA 7034 are within error, which suggests that the calculated average TP of 1450 ± 70°C doi:10.1002/2014JE004745. -

Chapter 5: Shock Metamorphism and Impact Melting
5. Shock Metamorphism and Impact Melting ❖❖❖ Shock-metamorphic products have become one of the diagnostic tools of impact cratering studies. They have become the main criteria used to identify structures of impact origin. They have also been used to map the distribution of shock-pressures throughout an impact target. The diverse styles of shock metamorphism include fracturing of crystals, formation of microcrystalline planes of glass through crystals, conversion of crystals to high-pressure polymorphs, conversion of crystals to glass without loss of textural integrity, conversion of crystals to melts that may or may not mix with melts from other crystals. Shock-metamorphism of target lithologies at the crater was first described by Barringer (1905, 1910) and Tilghman (1905), who recognized three different products. The first altered material they identified is rock flour, which they concluded was pulverized Coconino sandstone. Barringer observed that rock flour was composed of fragmented quartz crystals that were far smaller in size than the unaffected quartz grains in normal Coconino sandstone. Most of the pulverized silica he examined passed through a 200 mesh screen, indicating grain sizes <74 µm (0.074 mm), which is far smaller than the 0.2 mm average detrital grain size in normal Coconino (Table 2.1). Fairchild (1907) and Merrill (1908) also report a dramatic comminution of Coconino, although only 50% of Fairchild’s sample of rock flour passed through a 100 mesh screen, indicating grain sizes <149 µm. Heterogeneity of the rock flour is evident in areas where sandstone clasts survive within the rock flour. The rock flour is pervasive and a major component of the debris at the crater. -

Pigeonite Basalt 2109 Grams
12065 DRAFT Pigeonite Basalt 2109 grams Figure 1: Photo of 12065 showing numerous zap pits on rounded surface. Scale is in cm. NASA # S69-60591. Introduction Sample 12065 is a large rounded pigeonite basalt dated that near-surface olivine (Fo74) and some pyroxene at 3.16 ± 0.09 b.y. The outer surface is covered with settling could explain the variation in composition of micrometeorite pits on all sides (figure 1). some Apollo 12 basalts. Petrography Mineralogy 12065 is a variolitic basalt composed of pyroxene and Olivine: Olivine composition in 12065 ranges from olivine phenocrysts (figure 2) imbedded in a very fine Fo72-32 (Kushiro et al. 1971). matrix of feathery ilmenite, plagioclase and clinopyroxene (figure 3)(Reid 1971). Kushiro et al. Pyroxene: Hollister et al. (1971) and Kushiro et al. (1971) find that the fibrous pyroxene in 12065 is similar (1971) describe complex sector zoning of pyroxene to “quench pyroxenes” often found in quenching phenocrysts in 12065 (figure 4). Pigeonite cores are experiments. 12065 has a few percent void space. overgrown by subcalcic augite (Gay et al. 1971). Kushiro et al. report extreme Fe-enrichment in matrix Kushiro et al. (1971) used the bulk composition of pyroxene. Gay et al. report pyroxferroite with low Ca. 12065 to perform experiments leading to the conclusion Lunar Sample Compendium C Meyer 2005 Figure 2: Photomicrograph of thin section 12065 showing elongate pyroxene in variolitic groundmass. Scale is about 2 cm. NASA # S69-23378. Plagioclase: Plagioclase is An91 – An89 (Kushiro et al. 1971). Chemistry The chemical composition of 12065 has been reported Spinel: The Ti content of the Cr-spinel increases with by LSPET (1970), Maxwell et al. -

2713^7 Contents
MINERALS OF WASHINGTON, D.C. AND VICINITY by Lawrence R. Bernstein U. S. Geo^r^'ce.l Survey OPEN F-'::. r;.".r'0.?;r cer.-..: 2713^7 CONTENTS Introduction 1 Scope of report 4 Mineral collecting 5 Acknowledgments 6 Introduction 6a ITT3 Aclj.il1 -> ! T^______.___~^. -"» _«_____«..«_»«__.. " " .._.«__._.._*_.__._.,_.._.-. _>-.-- -_>-->.-..-. Q Triassic deposits 31 Mineral localities 38 District of Columbia : 38 IMavtrlWlCfci JT XClilUl a Tirl ~ __ ___« - - -_ -»-i-___ .__- _ __- - ________________ m~m~m~ m~ m~ «M » M* **A^J ^ Anne Arundel County 43 Baltimore County 45 Howard County - 74 Montgomery County 88 Prince Georges County 120 Virginia . 129 Arlington County 129 Fairfax County 131 Fauquier County 139 Loudoun County 143 Prince William County 149 Diabase quarries of northern Virginia 155 CAPTIONS Illustrations Plate 1. Mineral localities of Washington, B.C., and vicinity. Plate 2. Generalized geologic map of Washington. D.C. and vicinity, Plate 3. Mineral deposits and generalized geology of the Triassic rocks near Washington, D.C. List of Figures Figure 1. Index map showing region covered in this report. Stfaded area is covered in most detail. Figure 2. Block diagram of the Washington, D.C. region showing physiographic provinces and major geographic and geologic features. ITfgure -3. Coastal Plain deposits of Washington, D.C. and vicinity. Figure 4. Generalized cross section of a typical complex pegmatite of the Washington, D.C. area. Figure 5. Rhythmically.layered gabbro of the Baltimore Gabbro Com plex at Ilchester, Maryland. Figure 6. Triassic diabase dike forming a ridge north of Route 7 near Dranesville, Virginia.