RIDASCREEN® Campylobacter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Perforated Ulcers Shaleen Sathe, MS4 Christina Lebedis, MD CASE HISTORY
Perforated Ulcers Shaleen Sathe, MS4 Christina LeBedis, MD CASE HISTORY 54-year-old male with known history of hypertension presents with 2 days of acute onset abdominal pain, nausea, vomiting, and diarrhea, with periumbilical tenderness and abdominal distention on exam, without guarding or rebound tenderness. Labs, including CBC, CMP, and lipase, were unremarkable in the emergency department. Radiograph Perforated Duodenal Ulcer Radiograph of the chest in the AP projection shows large amount of free air under diaphragm (blue arrows), suggestive of intraperitoneal hollow viscus perforation. CT Perforated Duodenal Ulcer CT of the abdomen in the axial projection (I+, O-), at the level of the inferior liver edge, shows large amount of intraperitoneal free air (blue arrows) in lung window (b), and submucosal edema in the gastric antrum and duodenal bulb (red arrows), suggestive of a diagnosis of perforated bowel, most likely in the region of the duodenum. US Perforated Gastric Ulcer US of the abdomen shows perihepatic fluid (blue arrow) and free fluid in the right paracolic gutter (not shown), concerning for intraperitoneal pathology. Radiograph Perforated Gastric Ulcer Supine radiograph of the abdomen shows multiple air- filled dilated loops of large bowel, with air lucencies on both sides of the sigmoid colon wall (green arrows), consistent with Rigler sign and perforation. CT Perforated Gastric Ulcer CT of the abdomen in the axial (a) and sagittal (b) projections (I+, O-) shows diffuse wall thickening of the gastric body and antrum (green arrows) with an ulcerating lesion along the posterior wall of the stomach (red arrows), and free air tracking adjacent to the stomach (blue arrow), concerning for gastric ulcer perforation. -

Upper Gastrointestinal Endoscopy Prior to Laparoscopic Cholecystectomy: a Clinical Study at a Tertiary Care Centre in Central India
International Surgery Journal K olla V et al. Int Surg J. 2016 May;3(2):637-642 http://www.ijsurgery.com pISSN 2349-3305 | eISSN 2349-2902 DOI: http://dx.doi.org/10.18203/2349-2902.isj20161136 Research Article Upper gastrointestinal endoscopy prior to laparoscopic cholecystectomy: a clinical study at a tertiary care centre in central India Venkatesh Kolla, Neelam Charles*, Sanjay Datey, Devendra Mahor, Anand Gupta, Sanjeev Malhotra Department of General Surgery, SAMCPGI, Indore, MP, India Received: 11 January 2016 Revised: 21 February 2016 Accepted: 29 February 2016 *Correspondence: Dr. Neelam Charles, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Cholelithiasis is one of the most common problems encountered in surgery. It’s an immense challenge to discriminate between gastrointestinal symptoms due to gall stones or any other causes. These gastrointestinal symptoms have been related to gallstones but causal relationship has not been established yet, which is highly discouraging for the operating surgeon. An objectives of the study was to analyze the use of upper gastrointestinal endoscopy (UGE) as a pre-operative investigative tool in gallstone disease patients presenting with chronic dyspepsia. Methods: This prospective observational study was conducted on 216 patients at Department of Surgery at Aurobindo PG institute. The data collected from the patients included personal information, presenting signs and symptoms, investigations including USG, UGE finding, biopsy reports if present, medications, surgery details, any post-operative complications. -

Helicobacter Pylori (Hp) Infection of the Stomach and Relevant GI Symptoms
Name: DOB: PHN/ULI: RHRN: RefMD: Dr. RefMD Fax: RefDate: Date Today: March 8, 2016 CONFIRMATION: Referral Received Refractory TRIAGE CATEGORY: Enhanced Primary Care Pathway REFERRAL STATUS: CLOSED H. PYLORI Dear Dr. , The above-named patIent was referred to GI-CAT for further assessment of refractory Helicobacter pylori (Hp) infection of the stomach and relevant GI symptoms. Based on full review of your referral, It has been determined that management of this patient within the Enhanced Primary Care Pathway is appropriate, without need for specialist consultation at this time. ThIs clInIcal pathway has been developed by the Calgary Zone PrImary Care Network In partnershIp wIth the SectIon of Gastroenterology and Alberta Health ServIces. These local guidelines are based on best available clinical evIdence, and are practical in the prImary care setting. This package includes: 1. Focused summary of Hp relevant to prImary care 2. Summary of 2016 CanadIan AssocIatIon of Gastroenterology Guidelines for treatment of Helicobacter pylori 3. Review of your patient’s Hp treatment hIstory 4. Recommended next-round Hp treatment regImen 5. Checklist for your in-clinic followup of this patient This referral is CLOSED. If you would like to discuss this referral with a Gastroenterologist, call Specialist LINK, a dedicated GI phone consultation service, available 08:00-17:00 weekdays at 403-910-2551 or toll-free 1-855-387-3151. If your patient completes the Enhanced Primary Care Pathway and remains symptomatic or if your patient’s status or symptoms change, a new referral IndIcatIng ‘completed care pathway’ or ‘new InformatIon’ should be faxed to GI Central Access and TrIage at 403-944-6540. -

HYDROGEN & METHANE TESTING for IBS and Sugar Intolerances
phone: 1300 122 388 fax: 1300 122 399 www.breathtestlab.com.au PATIENT NAME: .......................................................................................................................................................................................................................... DOB: ........................................................................................................................................................ ADDRESS: ....................................................................................................................................................................................................................................................................................................................................................................................................................................... ...................................................................................................................................................................................................................................................................................................................................................................................................................................................................................... PHONE: ....................................................................................................................................................................... EMAIL: ................................................................................................................................................................................................................................. -

Dentistry – Surgery IV Year
Dentistry – Surgery IV year Which sign is not associated with peptic ulcer perforation: A 42-year-old male, with previous Sudden onset ulcer history and typical clinical picture of peptic ulcer perforation, on Cloiberg caps examination, in 4 hours after the Positive Blumberg sign beginning of the disease, discomfort in Free air below diaphragm on plain right upper quadrant, heart rate - abdominal film 74/min., mild abdominal wall muscles rigidity, negative Blumberg sign. Free Intolerable abdominal pain air below diaphragm on X-ray abdominal film. What is you diagnosis? Which ethiological factors causes Peptic ulcer recurrence peptic ulcer disease most oftenly? Acute cholecystitis Abdominal trauma, alimentary factor Chronic cholecystitis H. pylori, NSAID's Covered peptic ulcer perforation H. pylori, hyperlipidemia Acute appendicitis (subhepatic Drugs, toxins location) NSAID's, gastrinoma Most oftenly perforated peptic ulcers are located at: A 30-year-old male is operated on for peptic ulcer perforation, in 2,5 hours Posterior wall of antrum after the beginning of the disease. Fundus of stomach Which operation will be most efficient (radical operation for peptic ulcer)? Cardiac part of stomach Simple closure and highly selective Posterior wall of duodenal bulb vagotomy Anterior wall of duodenal bulb Simple closure Simple closure with a Graham patch Penetrated ulcer can cause such using omentum complications: 1). Abdominal abscess; Stomach resection 2). Portal pyelophlebitis; 3). Stomach- organ fistula; 4). Acute pancreatitis; 5). Antrumectomy Bleeding. 1, 2, 3; It has been abandoned as a method to treat ulcer disease 2, 3, 5; 1, 3, 5; A 42-year-old executive has refractory 3, 4, 5; chronic duodenal ulcer disease. -

Demographic, Chemical, and Helicobacter Pylori Positivity
Hindawi Canadian Journal of Gastroenterology and Hepatology Volume 2021, Article ID 3351352, 8 pages https://doi.org/10.1155/2021/3351352 Research Article Demographic, Chemical, and Helicobacter pylori Positivity Assessment in Different Types of Gallstones and the Bile in a Random Sample of Cholecystectomied Iranian Patients with Cholelithiasis Mohammad Bagher Jahantab ,1 Amir Abbas Safaripour ,1 Sajad Hassanzadeh ,2 and Mohammad Javad Yavari Barhaghtalab 1 1Department of General Surgery, Shahid Beheshti Hospital, Yasuj University of Medical Sciences, Yasuj, Iran 2Department of Internal Medicine, Imam Sajjad Hospital, Yasuj University of Medical Sciences, Yasuj, Iran Correspondence should be addressed to Mohammad Javad Yavari Barhaghtalab; [email protected] Received 6 May 2021; Revised 30 July 2021; Accepted 4 August 2021; Published 10 August 2021 Academic Editor: Masanao Nakamura Copyright © 2021 Mohammad Bagher Jahantab et al. *is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Background. *e occurrence of stones in the gallbladder or common bile duct and the symptoms and complications they cause is called gallstone disease. *e symptoms of gallstone disease range from mild, nonspecific symptoms to a severe right quadrant abdominal pain. Characteristics of gallstone types in an Iranian population have not been well studied before and there are very limited studies on the demographic pattern of stone types in our country, so this study is one of the first studies on its kind on the epidemiology of gallstone types in Iran. As information on chemical components of the stone will help in the management and prevention of gallstones, in this study, we aimed to do chemical component analysis of gallstones including cholesterol, bilirubin, and calcium. -

Helicobacter Pylori Education and Screening Study Sydnee Crankshaw1, Julia Butt1,2, Jennifer M
Crankshaw et al. BMC Gastroenterology (2020) 20:261 https://doi.org/10.1186/s12876-020-01405-w RESEARCH ARTICLE Open Access The Durham Initiative for Stomach Health (DISH): a pilot community-based Helicobacter pylori education and screening study Sydnee Crankshaw1, Julia Butt1,2, Jennifer M. Gierisch1,2,3, Nadine J. Barrett1,4,5, Sabrena Mervin-Blake5, Kevin Oeffinger1, Steven Patierno1,4,6,7, Valarie Worthy5, Ronald Godbee8 and Meira Epplein1,2,6* Abstract Background: Approximately 15% of all cancers are due to infection. The bacteria Helicobacter pylori is the single leading carcinogenic infectious agent and the main cause of stomach cancer. Prevalence of H. pylori, and, correspondingly, stomach cancer incidence and mortality, is significantly greater among African Americans than whites in the United States. In the present study, we conducted a pilot community-engaged H. pylori education and screening study in partnership with a predominantly African American church in Durham, North Carolina. Methods: Initially, we consulted with community advisory boards and convened stakeholder meetings with local community members and primary care physicians. We then developed this pilot study through an iterative collaboration with church partners. Our main outcomes were feasibility and acceptability as measured by participation in a one-day H. pylori screening initiative, and participation in follow-up for those who tested positive. We also sought to determine prevalence and determinants of active H. pylori infection in this population. Results: Community engagement informed the event logistics, messaging, educational materials provided, and follow-up plans. A total of 92 individuals participated in the primary study event, 25% of whom had a current H. -

Helicobacter Pylori Infection Prevalence and Histopathologic Findings in Laparoscopic Sleeve Gastrectomy
Obesity Surgery (2019) 29:3674–3679 https://doi.org/10.1007/s11695-019-04052-7 ORIGINAL CONTRIBUTIONS Helicobacter Pylori Infection Prevalence and Histopathologic Findings in Laparoscopic Sleeve Gastrectomy Gülay Turan1 & Servet Kocaöz2 Published online: 9 July 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Introduction Helicobacter pylori (H. pylori) is a type of bacteria that affects more than half of the world’s population and has been associated with gastritis. The relationship between H. pylori and obesity is controversial. Laparoscopic sleeve gastrectomy (LSG) is the most commonly used surgery for morbidly obese patients. The aim of this study was to investigate the rate of H. pylori in patients undergoing LSG. Methods Biopsy specimens of 32,743 patients who underwent esophagogastroduodenoscopy (EGD) and resection materials from 1257 patients who underwent LSG were examined histopathologically. The relationships between body mass index (BMI), age, gender, H. pylori infection, and intestinal metaplasia (IM) were investigated in patients with gastritis. Results In patients undergoing EGD, the association of H. pylori infection was found to be increased in males and the elderly (p < 0.001). The presence of gastritis and IM was significantly higher with H. pylori infection (p < 0.001 and p =0.001,respec- tively). H. pylori infection was significantly higher in patients over the age of 41 years (p < 0.001). There was no significant difference between the results of H. pylori before and after LSG surgery (p = 0.923). The presence of H. pylori together with gastritis and IM was found to be significant (p <0.001). Conclusions H. -

What Is New in Helicobacter Pylori Diagnosis. an Overview
Journal of Clinical Medicine Review What Is New in Helicobacter pylori Diagnosis. An Overview Maria Pina Dore 1,2,* and Giovanni Mario Pes 1 1 Dipartimento di Scienze Mediche, Chirurgiche e Sperimentali, University of Sassari, 07100 Sassari, Italy; [email protected] 2 Baylor College of Medicine, One Baylor Plaza, Houston, TX 77030, USA * Correspondence: [email protected]; Tel.: +39-079-229-886 Abstract: Helicobacter pylori infection remains one of the most prevalent infections worldwide, espe- cially in low-resource countries, and the major risk factor for peptic ulcer and gastric cancer. The “test-and-treat” strategy is recommended by several guidelines and consensus. The choice of testing method is based on patient age, presence of alarm signs and/or symptoms, use of non-steroidal anti-inflammatory drugs, as well as local availability, test reliability, and cost. Culture is the gold standard to detect H. pylori and, possibly, to perform susceptibility testing, however, it requires upper endoscopy and dedicated labs. Recent advances in molecular biology have provided new strategies in detecting infection and antimicrobial resistance without invasive tests. In this review we attempt to offer a comprehensive panorama on the new diagnostic tools and their potential use in clinical settings, in order to accomplish specific recommendations. Keywords: Helicobacter pylori; testing; antibiotic resistance; molecular techniques; artificial intelli- gence 1. Introduction Citation: Dore, M.P.; Pes, G.M. What Is New in Helicobacter pylori Helicobacter pylori infection can be essentially detected by invasive and non-invasive Diagnosis. An Overview. J. Clin. Med. tests. The choice of technique relies upon the patient’s needs. -

Helicobacter Pylori (H.Pylori) Primary Care Pathway
Helicobacter Pylori (H. pylori) Primary Care Pathway Quick links: Pathway primer Expanded details Advice options Patient pathway 1. Who should be tested for H. pylori? • Patients with dyspepsia symptoms • Patients with current or past gastric or duodenal ulcers or upper GI bleed • Patients with a first degree relative with a history of gastric cancer • First generation immigrants from Asia, Africa, Central and South America 2. Alarm features Dyspepsia symptoms plus one or more of following: • Age >60 with new and persistent symptoms (>3 months) Refer for • GI bleeding (melena or hematemesis) or anemia (if yes, do CBC, INR, PTT Yes consultation / as part of referral) • Progressive dysphagia gastroscopy • Persistent vomiting (not associated with cannabis use) • Unintended weight loss (≥5-10% of body weight over 6 months) • Personal history of peptic ulcer disease • First degree relative with history of esophageal or gastric cancer No 3. Diagnosis Local resources Follow Negative • Test using HpSAT or UBT dyspepsia • Before testing, patient must be off antibiotics x4 weeks pathway and off PPI at least 3 days Positive 4. Treatment • Round 1: CLAMET Quad or BMT Quad • Round 2 (if needed): CLAMET Quad or BMT Quad Yes • Round 3 (if needed): Levo Amox • Round 4 (if needed): Rif-Amox or refer to GI 5. Confirm eradication • HpSAT or UBT at least 4 weeks after finishing treatment Yes Symptoms • Before testing, patient must be off antibiotics x4 weeks persist and off PPI at least 3 days No No Pathway care 6. Treatment failure complete Or • Proceed to next round of treatment • Option to refer to GI after 3 failed treatment attempts Provider resources Background Patient resources Updated: April 2020 Pre-referral checklist Page 1 of 14 HELICOBACTER PYLORI (H. -
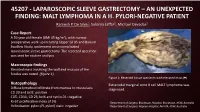
45207 - Laparoscopic Sleeve Gastrectomy – an Unexpected Finding: Malt Lymphoma in a H
45207 - LAPAROSCOPIC SLEEVE GASTRECTOMY – AN UNEXPECTED FINDING: MALT LYMPHOMA IN A H. PYLORI-NEGATIVE PATIENT Ramesh P. De Silva1, Sukaina Jaffar2, Michael Devadas2 Case Report A 59 year old female (BMI 45 kg/m2), with normal preoperative work up including Upper GI US and Barium Swallow Study, underwent an uncomplicated laparoscopic sleeve gastrectomy. The resected specimen was sent for routine analysis. Macroscopic findings Elevated mass involving the wall and mucosa of the fundus was noted (figure 1). Figure 1: Resected tissue specimen with elevated mass ( ) Histopathology Extranodal marginal zone B cell MALT lymphoma was Diffuse lymphoid infiltrate from mucosa to muscularis diagnosed. CD 20 and bcl2: positive CD5, CD10, CD 23, bcl6 and cyclin D1: negative Ki-67 proliferative index of 5% 1Department of Surgery, Blacktown Hospital, Blacktown, NSW, Australia Helicobacter pylori (H. pylori) stain: negative 2Department of Surgery, Nepean Hospital, Penrith, NSW, Australia Post Operative Management The case was discussed at the local MDT meeting and the patient was reviewed by a Haematologist who did not find evidence for lymphoproliferative disorder. Post operative urea breath test was negative for H. pylori. The patient was placed on a twice yearly endoscopic surveillance program. At 1 year follow up, the patient had lost 31 kg, and had no post operative complications or lymphoma. Discussion • Gastric MALT lymphoma often presents with non-specific symptoms such as abdominal discomfort, vomiting and diarrhoea. Diagnosis is often incidental on endoscopic biopsy1. • It is overwhelmingly linked to concurrent Helicobacter pylori infection with infection present in 70-90% of patients with MALT lymphoma1. • Routine surveillance of H. -

E83 Quality of Life Following Liver Transplantation
3rd UEGWOslo 1994 A19 cycline (OT), and metronidazole (M). Methods: Treatment 1(101 patients): B Internal consistency of the variables as assessed by Cronbach's a was 5 ml, OT 500 mg, and M 200 mg all q.i.d. for 14 days, Treatment 11(60 pa- found to be >0.8 in all dimensions of QL. A strong correlation with the num- tients): B 10 ml and OT 750 mg q.i.d., M 400 mg t.i.d. for 7 days, Treatment ber of associated diseases was found and 14-28 day test-retest reliability in Gut: first published as 10.1136/gut.35.4_Suppl.A19 on 1 January 1994. Downloaded from III (145 patients): B 10 ml and OT 500 mg q.i.d., M 400 mg t.i.d. for 10 days. stable patients was r = 0.7. Discrimination among different stages of disease Gastroscopy and 14C-urea breath test were performed 4 weeks and 1 year was higher when compared with the SF 36. Results in different areas of QL after cessation of therapy. Results: H. pylori eradication rates at 4 weeks were of the developed LSQL are shown in the table. 87.9%, 82.1%, and 95.7% for Treatment I, lIand l1l, respectively, according to the breath test. Having re-examined 100 patients after one year with both gas- Liver-Dx Pre-OLT Post-OLT P-Value troscopy and breath test without finding any active ulcer in asymptomatic, H. Cognitive Symptoms 71.0% 74.0% 78.9% NS pylori negative patients, we changed the protocol and did breath test only at Somatic Symptoms 65.9% 64.3% 79.7% 0.00001 Physical Function 62.3% 48.6% 72.9% 0.0062 one year in these patients.