A Comprehensive Review on Plant-Derived Mucilage: Characterization, Functional Properties, Applications, and Its Utilization for Nanocarrier Fabrication
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Central Composite Design for Optimizing the Biosynthesis Of
www.nature.com/scientificreports OPEN Central Composite Design for Optimizing the Biosynthesis of Silver Nanoparticles using Plantago major Extract and Investigating Antibacterial, Antifungal and Antioxidant Activity Ghazal Nikaeen1, Saeed Yousefnejad1 ✉ , Samane Rahmdel2, Fayezeh Samari3 & Saeideh Mahdavinia1 Central composite design (CCD) was applied to optimize the synthesis condition of silver nanoparticles (AgNPs) using the extract of Plantago major (P. major) seeds via a low cost and single-step process. The aqueous seed extract was applied as both reducing element and capping reagent for green production of AgNPs. Five empirical factors of synthesis including temperature (Temp), pH, volume of P. major extract (Vex), volume of AgNO3 solution (VAg) and synthesis time were used as independent variables of model and peak intensity of Surface Plasmon Resonance (SPR) originated from NPs as the dependent variable. The predicted optimal conditions was determined to be: Temp = 55 °C, pH = 9.9,Vex = 1.5 mL, VAg = 30 mL, time = 60 min. The characterization of the prepared AgNPs at these optimum conditions was conducted by Fourier transform infrared spectroscopy (FTIR), dynamic light scattering (DLS), transmission electron microscopy (TEM) and X-ray difraction (XRD) to determine the surface bio- functionalities. Bio-activity of these AgNPs against bacteria and fungi were evaluated based on its assay against Micrococcus luteus, Escherichia coli and Penicillium digitatum. Furthermore, antioxidant capacity of these NPs was checked using the ferric reducing antioxidant power (FRAP) assay. Nanotechnology is an important feld of modern research which has been the principal of various technologies and main innovations; and is expected to be the basis of many other outstanding innovations in future. -

View PDF Version
Food & Function View Article Online PAPER View Journal | View Issue Chia seed mucilage – a vegan thickener: isolation, tailoring viscoelasticity and rehydration Cite this: Food Funct., 2019, 10, 4854 Linda Brütsch, Fiona J. Stringer, Simon Kuster, Erich J. Windhab and Peter Fischer * Chia seeds and their mucilage gels provide a nutritionally and functionally promising ingredient for the food and pharmaceutical industry. Application and utilization of the gel remain limited due to the tightly adhesion of the mucilage to the seeds, which affects the organoleptic properties, control of concen- tration and structuring possibilities. To exploit the full potential of chia mucilage gels as a functional ingre- dient calls for separation and purification of the gel. Herein, the gel was extracted by centrifugation and characterized rheologically and microscopically to link the viscoelastic properties to the structural pro- perties. Subsequently, the gel was dried employing three different methods for facilitated storage and prolonged shelf life. The dried gels were readily soluble and its viscoelastic properties were fully regener- Creative Commons Attribution 3.0 Unported Licence. ated upon rehydration demonstrating its potential to envisage industrial applications. The viscoelastic chia mucilage demonstrated shear-thinning behavior with complete relaxation upon stress removal. The gel’s Received 26th January 2018, elasticity was enhanced with increasing mucilage concentration resulting in a highly tunable system. The Accepted 13th July 2019 extractable and rehydratable functional chia gel is a viable candidate as additive for the development of DOI: 10.1039/c8fo00173a products requiring specific viscoelastic properties. Addition of the gel enhances the nutritional profile rsc.li/food-function without interfering with the organoleptic properties. -

Mucilage Problem in the Semi-Enclosed Seas: Recent Outbreak in the Sea of Marmara
ISSN: 2148-9173 Vol: Issue:4 December 2021 ,QWHUQDWLRQDO-RXUQDORI(QYLURQPHQWDQG*HRLQIRUPDWLFV ,-(*(2 LVDQLQWHUQDWLRQDO PXOWLGLVFLSOLQDU\SHHUUHYLHZHGRSHQDFFHVVMRXUQDO Mucilage Problem in the Semi-Enclosed Seas: Recent Outbreak in the Sea of Marmara Başak SAVUN-HEKİMOĞLU, Cem GAZİOĞLU &KLHILQ(GLWRU 3URI'U&HP*D]LR÷OX &R(GLWRUV 3URI'U'XUVXQ=DIHUùHNHU3URI'UùLQDVL.D\D 3URI'U$\úHJO7DQÕNDQG$VVLVW3URI'U9RONDQ'HPLU (GLWRULDO&RPPLWWHH December $VVRc3URI'U$EGXOODK$NVX 75 $VVLW3URI'U8÷XU$OJDQFÕ 75 3URI'U%HGUL$OSDU 75 Assoc. Prof. Dr. Aslı Aslan (US), 3URI'U/HYHQW%DW 75 3URI'U3DXO%DWHV 8. øUúDG%D\ÕUKDQ 75 3URI'U%OHQW %D\UDP 75 3URI'U/XLV0%RWDQD (6 3URI'U1XUD\dD÷ODU 75 3URI'U6XNDQWD'DVK ,1 'U6RRILD7 (OLDV 8. 3URI'U$(YUHQ(UJLQDO 75 $VVRF3URI'U&QH\W(UHQR÷OX 75 'U'LHWHU)ULWVFK '( 3URI 'UdL÷GHP*|NVHO 75 3URI'U/HQD+DORXQRYD &= 3URI'U0DQLN.DOXEDUPH ,1 'U+DNDQ.D\D 75 $VVLVW3URI'U6HUNDQ.NUHU 75 $VVRF3URI'U0DJHG0DUJKDQ\ 0< 3URI'U0LFKDHO0HDGRZV =$ 3URI 'U 1HEL\H 0XVDR÷OX 75 3URI 'U 0DVDIXPL 1DNDJDZD -3 3URI 'U +DVDQ g]GHPLU 75 3URI 'U &KU\VV\3RWVLRX *5 3URI'U(URO6DUÕ 75 3URI'U0DULD3DUDGLVR ,7 3URI'U3HWURV3DWLDV *5 3URI'U (OLI6HUWHO 75 3URI'U1NHW6LYUL 75 3URI'U)VXQ%DOÕNùDQOÕ 75 3URI'U8÷XUùDQOÕ 75 'X\JXhONHU 75 3URI'U6H\IHWWLQ7Dú 75 $VVRF3URI'UgPHU6XDW7DúNÕQ TR Assist. Prof. Dr. Tuba Ünsal (TR), Dr. Manousos Valyrakis (UK), 'UøQHVH9DUQD /9 'U3HWUD9LVVHU 1/ 3URI'U6HOPDhQO 75 Assoc. Prof. Dr. Oral Yağcı (TR), 3URI'U0XUDW<DNDU 75 Assoc. Prof. Dr. İ. Noyan Yılmaz (AU); $VVLW3URI'U6LEHO=HNL 75 $EVWUDFWLQJ DQG ,QGH[LQJ 75 ',=,1 '2$- ,QGH[ &RSHUQLFXV 2$-, 6FLHQWLILF ,QGH[LQJ 6HUYLFHV ,QWHUQDWLRQDO 6FLHQWLILF ,QGH[LQJ-RXUQDO)DFWRU*RRJOH6FKRODU8OULFK V3HULRGLFDOV'LUHFWRU\:RUOG&DW'5-,5HVHDUFK%LE62%,$' International Journal of Environment and Geoinformatics 8(4): 402-413 (2021) Review Article Mucilage Problem in the Semi-Enclosed Seas: Recent Outbreak in the Sea of Marmara Başak Savun-Hekimoğlu* , Cem Gazioğlu Institute of Marine Sciences and Management, İstanbul University, İstanbul, Turkey * Corresponding author: B. -

Xylan Utilization in Human Gut Commensal Bacteria Is Orchestrated by Unique Modular Organization of Polysaccharide-Degrading Enzymes
Xylan utilization in human gut commensal bacteria is orchestrated by unique modular organization of polysaccharide-degrading enzymes Meiling Zhanga,b,c,1,2, Jonathan R. Chekand,1, Dylan Dodda,b,e,1,3, Pei-Ying Hongc,4, Lauren Radlinskia,b,e, Vanessa Revindrana,b, Satish K. Nairb,d,5, Roderick I. Mackiea,b,c,5, and Isaac Canna,b,c,e,5 aEnergy Biosciences Institute, bInstitute for Genomic Biology, and Departments of cAnimal Sciences, dBiochemistry, and eMicrobiology, University of Illinois at Urbana-Champaign, Urbana, IL 61801 Edited by Arnold L. Demain, Drew University, Madison, NJ, and approved July 29, 2014 (received for review April 19, 2014) Enzymes that degrade dietary and host-derived glycans represent One of the most important functional roles specific to the lower the most abundant functional activities encoded by genes unique gut microbiota is the degradation and fermentation of dietary fiber to the human gut microbiome. However, the biochemical activities that is resistant to digestion by human enzymes. The enzymes in- of a vast majority of the glycan-degrading enzymes are poorly volved in breaking down polysaccharides are members of carbo- understood. Here, we use transcriptome sequencing to under- hydrate active enzymes (CAZymes) and are categorized into stand the diversity of genes expressed by the human gut bacteria families of carbohydrate-binding modules (CBMs), carbohydrate Bacteroides intestinalis and Bacteroides ovatus grown in monocul- esterases (CEs), glycoside hydrolases (GHs), glycoside transferases ture with the abundant dietary polysaccharide xylan. The most (GTs), or polysaccharide lyases (PLs) (14). The CAZy gene cat- highly induced carbohydrate active genes encode a unique glycoside alog of the human genome pales in comparison with that of the gut hydrolase (GH) family 10 endoxylanase (BiXyn10A or BACINT_04215 microbiota, which exceeds the total number of human CAZymes and BACOVA_04390) that is highly conserved in the Bacteroidetes by at least 600-fold (15). -

Mucilage in Yellow Mustard (Brassica Hirta) Seeds
Food Structure Volume 5 Number 1 Article 17 1986 Mucilage in Yellow Mustard (Brassica Hirta) Seeds I. R. Siddiqui S. H. Yiu J. D. Jones M. Kalab Follow this and additional works at: https://digitalcommons.usu.edu/foodmicrostructure Part of the Food Science Commons Recommended Citation Siddiqui, I. R.; Yiu, S. H.; Jones, J. D.; and Kalab, M. (1986) "Mucilage in Yellow Mustard (Brassica Hirta) Seeds," Food Structure: Vol. 5 : No. 1 , Article 17. Available at: https://digitalcommons.usu.edu/foodmicrostructure/vol5/iss1/17 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Food Structure by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. FOOD MICROSTRUCTURE, Vol. 5 (1986), pp. 157-162 0730-54 19/86$ I. 00 •. OS SEM, Inc., AMF O'Hare (Chicago), IL 60666- 0507 U.S.A . MUCILAGE IN YELLOW MUSTARD (BRASSICA H I RT A) SEEDS I. R. Siddiqui. S. H. V!u, J . 0. Jones, and M. Kal.ib Food Resea r ch Cen tre, Research Branch, Agriculture Canada Ottawa, On tario, Canada KlA OC6 Introduction Release of mucJ !age fr011 yellow JDUstard (Brass lea Seeds of the genus Brass i ca arc known to contain hirta, also IOlown as Sinapis alba) seed coats (hulls) varying a.ounts of •ucilage. The 110cf I age Is of particu was studied by optical and scanning electron •icroscopy. lar Importance in -.!stard seeds because it contributes Mi crographs were obtained of the aucilage which had to the consistency of prepared lllllstard (Weber et a l. -
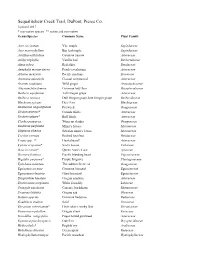
Sequalitchew Creek Trail Plant List
Sequalitchew Creek Trail, DuPont, Pierce Co. Updated 2017 * non-native species ** native and non-native Genus/Species Common Name Plant Family Acer circinatum Vine maple Sapindaceae Acer macrophyllum Big leaf maple Sapindaceae Achillea millefolium Common yarrow Asteraceae Achlys triphylla Vanilla leaf Berberidaceae Alnus rubra Red alder Betulaceae Anaphalis margaritacea Pearly everlasting Asteraceae Arbutus menziesii Pacific madrone Ericaceae Artemisia suksdorfii Coastal wormwood Asteraceae Asarum caudatum Wild ginger Aristolochiaceae Athyrium felix-femina Common lady fern Dryopteridaceae Berberis aquifolium Tall Oregon grape Asteraceae Berberis nervosa Dull Oregon grape, low Oregon grape Berberidaceae Blechnum spicant Deer fern Blechnaceae Chamerion angustifolium Fireweed Onagraceae Cirsium arvense* Canada thistle Asteraceae Cirsium vulgare* Bull thistle Asteraceae Clarkia purpurea Winecup clarkia Onagraceae Claytonia perfoliata Miner's lettuce Montiaceae Claytonia siberica Siberian miner's lettuce Montiaceae Corylus cornuta Beaked hazelnut Betulaceae Crepis spp. ?* Hawksbeard? Asteraceae Cytisus scoparius* Scot's broom Fabaceae Daucus carota* Queen Anne's Lace Apiaceae Dicentra formosa Pacific bleeding heart Papaveraceae Digitalis purpurea* Purple foxglove Plantaginaceae Epilobium minutum Threadstem fireweed Onagraceae Equisetum arvense Common horsetail Equisetaceae Equisetum telmateia Giant horsetail Equisetaceae Eriophyllum lanatum Oregon sunshine Asteraceae Erythronium oregonum White fawn lily Liliaceae Frangula purshiana Cascara, -

Lyonia Preserve Plant Checklist
Lyonia Preserve Plant Checklist Volusia County, Florida Aceraceae (Maple) Asteraceae (Aster) Red Maple Acer rubrum Bitterweed Helenium amarum Blackroot Pterocaulon virgatum Agavaceae (Yucca) Blazing Star Liatris sp. Adam's Needle Yucca filamentosa Blazing Star Liatris tenuifolia Nolina Nolina brittoniana Camphorweed Heterotheca subaxillaris Spanish Bayonet Yucca aloifolia Cudweed Gnaphalium falcatum Dog Fennel Eupatorium capillifolium Amaranthaceae (Amaranth) Dwarf Horseweed Conyza candensis Cottonweed Froelichia floridana False Dandelion Pyrrhopappus carolinianus Fireweed Erechtites hieracifolia Anacardiaceae (Cashew) Garberia Garberia heterophylla Winged Sumac Rhus copallina Goldenaster Pityopsis graminifolia Goldenrod Solidago chapmanii Annonaceae (Custard Apple) Goldenrod Solidago fistulosa Flag Paw paw Asimina obovata Goldenrod Solidago spp. Mohr's Throughwort Eupatorium mohrii Apiaceae (Celery) Ragweed Ambrosia artemisiifolia Dollarweed Hydrocotyle sp. Saltbush Baccharis halimifolia Spanish Needles Bidens alba Apocynaceae (Dogbane) Wild Lettuce Lactuca graminifolia Periwinkle Catharathus roseus Brassicaceae (Mustard) Aquifoliaceae (Holly) Poorman's Pepper Lepidium virginicum Gallberry Ilex glabra Sand Holly Ilex ambigua Bromeliaceae (Airplant) Scrub Holly Ilex opaca var. arenicola Ball Moss Tillandsia recurvata Spanish Moss Tillandsia usneoides Arecaceae (Palm) Saw Palmetto Serenoa repens Cactaceae (Cactus) Scrub Palmetto Sabal etonia Prickly Pear Opuntia humifusa Asclepiadaceae (Milkweed) Caesalpinceae Butterfly Weed Asclepias -

Fort Ord Natural Reserve Plant List
UCSC Fort Ord Natural Reserve Plants Below is the most recently updated plant list for UCSC Fort Ord Natural Reserve. * non-native taxon ? presence in question Listed Species Information: CNPS Listed - as designated by the California Rare Plant Ranks (formerly known as CNPS Lists). More information at http://www.cnps.org/cnps/rareplants/ranking.php Cal IPC Listed - an inventory that categorizes exotic and invasive plants as High, Moderate, or Limited, reflecting the level of each species' negative ecological impact in California. More information at http://www.cal-ipc.org More information about Federal and State threatened and endangered species listings can be found at https://www.fws.gov/endangered/ (US) and http://www.dfg.ca.gov/wildlife/nongame/ t_e_spp/ (CA). FAMILY NAME SCIENTIFIC NAME COMMON NAME LISTED Ferns AZOLLACEAE - Mosquito Fern American water fern, mosquito fern, Family Azolla filiculoides ? Mosquito fern, Pacific mosquitofern DENNSTAEDTIACEAE - Bracken Hairy brackenfern, Western bracken Family Pteridium aquilinum var. pubescens fern DRYOPTERIDACEAE - Shield or California wood fern, Coastal wood wood fern family Dryopteris arguta fern, Shield fern Common horsetail rush, Common horsetail, field horsetail, Field EQUISETACEAE - Horsetail Family Equisetum arvense horsetail Equisetum telmateia ssp. braunii Giant horse tail, Giant horsetail Pentagramma triangularis ssp. PTERIDACEAE - Brake Family triangularis Gold back fern Gymnosperms CUPRESSACEAE - Cypress Family Hesperocyparis macrocarpa Monterey cypress CNPS - 1B.2, Cal IPC -

Insights Into the Ph-Dependent, Extracellular Sucrose Utilization and Concomitant Levan Formation by Gluconobacter Albidus TMW 2.1191
Antonie van Leeuwenhoek (2020) 113:863–873 https://doi.org/10.1007/s10482-020-01397-3 (0123456789().,-volV)( 0123456789().,-volV) ORIGINAL PAPER Insights into the pH-dependent, extracellular sucrose utilization and concomitant levan formation by Gluconobacter albidus TMW 2.1191 Frank Jakob . Clara Gebrande . Regina M. Bichler . Rudi F. Vogel Received: 18 December 2019 / Accepted: 20 February 2020 / Published online: 4 March 2020 Ó The Author(s) 2020 Abstract Many bacteria and archaea produce the The glucose release and formation of high molecular polydisperse fructose polymer levan from sucrose weight levans ([ 3.5 kDa) from 0.1 M initial sucrose upon biofilm formation via extracellular levansucrases was comparable between pH * 4.3–5.7 using equal (EC 2.4.1.10). We have investigated levansucrase- amounts of released levansucrase. Hence, this type of release and -activities as well as molecular size of the levansucrase appears to be structurally adapted to levan formed by the acetic acid bacterium Glu- changes in the extracellular pH and to exhibit a similar conobacter albidus TMW 2.1191 at varying environ- total activity over a wide acidic pH range, while it mental pH conditions to obtain insight in the produced higher amounts of larger levan molecules at ecological role of its constitutively expressed levan- higher production pH and sucrose concentrations. sucrase and the produced levan. A buffer system was These findings indicate the physiological adaptation of established enabling the recovery of levansucrase- G. albidus TMW 2.1191 to efficient colonisation of containing supernatants from preincubated cell sus- sucrose-rich habitats via released levansucrases pensions at pH 4.3–pH 5.7. -

Estrogen and Thyroid Hormone Receptor Activation by Medicinal Plants from Bahia, Brazil
medicines Article Estrogen and Thyroid Hormone Receptor Activation by Medicinal Plants from Bahia, Brazil Luã Tainã Costa Reis 1 ID , Magnus Régios Dias da Silva 2, Silvia Lima Costa 3, Eudes da Silva Velozo 4, Ronan Batista 5 ID and Suzana Telles da Cunha Lima 1,* ID 1 Laboratory of Bioprospection and Biotechnology (LaBBiotec), Institute of Biology, Federal University of Bahia (UFBA), Barão de Jeremoabo Street, 147-Ondina, Salvador, BA 40170-115, Brazil; [email protected] 2 Laboratory of Molecular and Translational Endocrinology, Department of Medicine, Federal University of São Paulo (UNIFESP), R. Sena Madureira, 1500-Vila Clementino, São Paulo, SP 04021-001, Brazil; [email protected] 3 Laboratory of Neurochemistry and Cell Biology, Department of Biofunction, Institute of Health Sciences, Federal University of Bahia (UFBA), Reitor Miguel Calmon Avenue, 1272-Canela, Salvador, BA 40231-300, Brazil; [email protected] 4 Laboratory of Research in Materia Medica, Department of Medicament, Faculty of Pharmacy, Federal University of Bahia (UFBA), Barão de Jeremoabo Street, 147-Ondina, Salvador, BA 40170-115, Brazil; [email protected] 5 Department of Organic Chemistry, Institute of Chemistry, Federal University of Bahia (UFBA), Barão de Jeremoabo Street, 147-Ondina, Salvador, BA 40170-115, Brazil; [email protected] * Correspondence: [email protected]; Tel.: +55-71-987-938-847 Received: 23 December 2017; Accepted: 11 January 2018; Published: 15 January 2018 Abstract: Background: A number of medicinal plants are traditionally used for metabolic disorders in Bahia state, Brazil. The aim of this study was to evaluate the estrogen receptor (ER) and thyroid receptor (TR) activation of crude extracts prepared from 20 plants. -

Plant Essential Oils and Formamidines As Insecticides/Acaricides: What Are the Molecular Targets? Wolfgang Blenau, Eva Rademacher, Arnd Baumann
Plant essential oils and formamidines as insecticides/acaricides: what are the molecular targets? Wolfgang Blenau, Eva Rademacher, Arnd Baumann To cite this version: Wolfgang Blenau, Eva Rademacher, Arnd Baumann. Plant essential oils and formamidines as in- secticides/acaricides: what are the molecular targets?. Apidologie, Springer Verlag, 2012, 43 (3), pp.334-347. 10.1007/s13592-011-0108-7. hal-01003531 HAL Id: hal-01003531 https://hal.archives-ouvertes.fr/hal-01003531 Submitted on 1 Jan 2012 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Apidologie (2012) 43:334–347 Review article * INRA, DIB and Springer-Verlag, France, 2011 DOI: 10.1007/s13592-011-0108-7 Plant essential oils and formamidines as insecticides/ acaricides: what are the molecular targets? 1 2 3 Wolfgang BLENAU , Eva RADEMACHER , Arnd BAUMANN 1Institut für Bienenkunde (Polytechnische Gesellschaft), Goethe-Universität Frankfurt am Main, Karl-von-Frisch-Weg 2, 61440 Oberursel, Germany 2Institute of Biology, Freie Universität Berlin, 14195 Berlin, Germany 3Institute of Complex Systems—Cellular Biophysics-(ICS-4), Forschungszentrum Jülich, 52425 Jülich, Germany Received 16 May 2011 – Revised 29 August 2011 – Accepted 21 October 2011 Abstract – The parasitic mite Varroa destructor is the main cause of the severe reduction in beekeeping during the last few decades. -

Edible Flowers — Swansons Nursery - Seattle's Favorite Garden Store Since 1924
edible flowers — Swansons Nursery - Seattle's Favorite Garden Store Since 1924 << BACK TO NW GARDENING TIPS EDIBLE FLOWERS FOR THE NORTHWEST GARDENER Edible flowers are a lot of fun to experiment with, yet little (and much contradictory) information exists about them. This list excludes all known poisonous and questionable flowers as well as most tropical flowers and some edible flowers with little culinary merit. Please note that this list pertains only to the edibility of the flower portion of the plant. Finally, never eat any plant or flower you cannot identify with certainty. Note: Treat eating edible flowers as you might mushrooms. Different people have different sensitivities—try a small piece to check out your personal reaction. Anise Hyssop - Agastache foeniculum Arugula - Erusca vesicaria Basil - Ocimum basilicum Batchelor Button - Centaurea cyanus Bee Balm - Monarda didyma Begonia - Begonia hybrid Borage - Borago officinalis Brassicas - Brassica spp. Calendula - Calendula officinalis Clove Pink - Dianthus caryophyllus Chamomile - Matricaria recutita Chervil - Anthriscus cerefolium Chive - Allium schoenorasum https://www.swansonsnursery.com/edible-flowers[1/24/2020 8:53:33 AM] edible flowers — Swansons Nursery - Seattle's Favorite Garden Store Since 1924 Garlic Chives - Allium tuberosum Chrysanthemums - Chrysanth. x morifolium Citrus Blossoms - Citrus limon, C. sinensis Clover, Red - Trifolium pratense Coriander - Coriandrum sativum Cress - Lepidium sativum Daisy, English - Bellis perennis Dandelion - Taraxacum officinale Day Lily - Hemerocallis fulva Dill - Anethum graveolens Elderberry - Sambucus canadensis ALL ELDER FLOWERS ARE EDIBLE. BLUE ELDER BERRIES ARE EDIBLE. RED ELDER BERRIES ARE POISONOUS! Fennel - Foeniculum vulgare Fuchsia - Fuchsia hybrid Garlic Mustard - Allaria petiolata Geranium, Scented - Pelargoniums Gladiolas - Gladiolus spp. DO NOT EAT GLADIOLUS GANDAVENSIS.