Glycomark Compendium of Evidence
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 7: Monogenic Forms of Diabetes
CHAPTER 7 MONOGENIC FORMS OF DIABETES Mark A. Sperling, MD, and Abhimanyu Garg, MD Dr. Mark A. Sperling is Emeritus Professor and Chair, University of Pittsburgh, Department of Pediatrics, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA. Dr. Abhimanyu Garg is Professor of Internal Medicine and Chief of the Division of Nutrition and Metabolic Diseases at University of Texas Southwestern Medical Center, Dallas, TX. SUMMARY Types 1 and 2 diabetes have multiple and complex genetic influences that interact with environmental triggers, such as viral infections or nutritional excesses, to result in their respective phenotypes: young, lean, and insulin-dependence for type 1 diabetes patients or older, overweight, and often manageable by lifestyle interventions and oral medications for type 2 diabetes patients. A small subset of patients, comprising ~2%–3% of all those diagnosed with diabetes, may have characteristics of either type 1 or type 2 diabetes but have single gene defects that interfere with insulin production, secretion, or action, resulting in clinical diabetes. These types of diabetes are known as MODY, originally defined as maturity-onset diabetes of youth, and severe early-onset forms, such as neonatal diabetes mellitus (NDM). Defects in genes involved in adipocyte development, differentiation, and death pathways cause lipodystrophy syndromes, which are also associated with insulin resistance and diabetes. Although these syndromes are considered rare, more awareness of these disorders and increased availability of genetic testing in clinical and research laboratories, as well as growing use of next generation, whole genome, or exome sequencing for clinically challenging phenotypes, are resulting in increased recognition. A correct diagnosis of MODY, NDM, or lipodystrophy syndromes has profound implications for treatment, genetic counseling, and prognosis. -

Myalept® (Metreleptin)
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2021 P 2032-11 Program Prior Authorization/Medical Necessity Medication Myalept® (metreleptin) P&T Approval Date 5/2014, 7/2014, 8/2014, 7/2015, 6/2016, 5/2017, 5/2018, 5/2019, 5/2020, 5/2021 Effective Date 8/1/2021; Oxford only: 8/1/2021 1. Background: Myalept (metreleptin) is a leptin analog indicated as an adjunct to diet as replacement therapy to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy.1 Myalept is available only through a restricted distribution program under a Risk Evaluation and Mitigation Strategy (REMS), called the Myalept REMS program, because of the risks associated with the development of anti-metreleptin antibodies that neutralize endogenous leptin and the risk of lymphoma. 2. Coverage Criteria: A. Initial Authorization 1. Myalept will be approved based on all of the following criteria: a. Diagnosis of congenital or acquired generalized lipodystrophy associated with leptin deficiency -AND- b. Myalept is being used as an adjunct to diet modification -AND- c. Prescribed by an endocrinologist -AND- d. Documentation demonstrates that patient has at least one of the following: (1) Diabetes mellitus or insulin resistance with persistent hyperglycemia (HgbA1C > 7.0) despite both of the following: (a) Dietary intervention (b) Optimized insulin therapy at maximum tolerated doses © 2021 UnitedHealthcare Services, Inc. 1 -OR- (2) Persistent hypertriglyceridemia (TG > 250) despite both of the following: (a) Dietary intervention (b) Optimized therapy with at least two triglyceride-lowering agents from different classes (e.g., fibrates, statins) at maximum tolerated doses Authorization will be issued for 12 months B. -
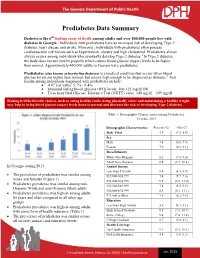
2015 Prediabetes Data Summary
The Georgia Department of Public Health Prediabetes Data Summary Diabetes is the 6th leading cause of death among adults and over 800,000 people live with diabetes in Georgia.1 Individuals with prediabetes have an increased risk of developing Type 2 diabetes, heart disease and stroke. Moreover, individuals with prediabetes often possess cardiovascular risk factors such as hypertension, obesity and high cholesterol. Prediabetes almost always occurs among individuals who eventually develop Type 2 diabetes.2 In Type 2 diabetes, the body does not use insulin properly which causes blood glucose (sugar) levels to be higher than normal. Approximately 450,000 adults in Georgia have prediabetes. Prediabetes (also known as borderline diabetes) is a medical condition that occurs when blood glucose levels are higher than normal, but are not high enough to be diagnosed as diabetes.3 Test results among individuals diagnosed with prediabetes include: A1C test value: 5.7% - 6.4% Impaired fasting blood glucose (IFG) levels: 100-125 mg/dl OR Two- hour Oral Glucose Tolerance Test (OGTT) value: 140 mg/dl – 199 mg/dl Making healt hy lifestyle choices, such as eating healthy foods, being physically active and maintaining a healthy weight, may help to bring blood glucose (sugar) levels down to normal and decrease the risk of developing Type 2 diabetes Table 1. Demographic Characteristics among Prediabetics, Georgia, 2013 Demographic Characteristics Percent (%) 95% CI State Total 7.9 (7.2, 8.9) Sex Male 7.8 (6.8, 9.5) Female 7.9 (6.9, 9.1) Race/Ethnicity -

Not Every Diabetic Child Has Type 1 Diabetes Mellitus Nem Toda Criança Diabética É Tipo 1 Thais Della Manna*
0021-7557/07/83-05-Supl/S178 Jornal de Pediatria Copyright © 2007 by Sociedade Brasileira de Pediatria ARTIGO DE REVISÃO Not every diabetic child has type 1 diabetes mellitus Nem toda criança diabética é tipo 1 Thais Della Manna* Resumo Abstract Objetivo: Apesar de o diabetes melito tipo 1 de origem Objective: Although it is type 1 diabetes mellitus of autoimmune autoimune ser o mais prevalente na infância e adolescência, outras origin that is most prevalent in childhood and adolescence, other formas de diabetes também podem acometer essa população, forms of diabetes can also affect this population, resulting in different implicando em prognóstico e tratamentos diferentes. prognosis and treatment. Fontes dos dados: Foram utilizadas informações através de Sources: Information was obtained by means of a bibliographic revisão bibliográfica realizada por busca direta de artigos científicos review, carried out by running searches for scientific articles in the nas bases de dados MEDLINE e LILACS, além de publicações clássicas MEDLINE and LILACS databases, in addition to classic publications referentes ao tema, sendo escolhidas as mais representativas. on the subject, with the most representative being chosen. Síntese dos dados: Este artigo discute os mecanismos Summary of the findings: This article discusses the fisiopatológicos, quadro clínico e tratamento das diversas formas de pathophysiological mechanisms, clinical presentation and treatment diabetes que acometem a faixa etária pediátrica, como diabetes of the various forms of diabetes -

Putting Medications in Perspective for Chronic Weight Management
05/06/2019 1 Donna H. Ryan, MD Professor Emerita Pennington Biomedical Research Center Baton Rouge, LA, USA The Role of Pharmacology in Weight Management: Putting Medications in Perspective for Chronic Weight Management 1 Disclosures • Consulting fee: Amgen, Gila Therapeutics, IFA Celtic, Novo Nordisk, Bausch Health, Real Appeal, Sanofi, Quintiles Novella, Paul Hastings, Simmons and Simmons, ReDesign Health, KVK Tech • Speakers bureau: Novo Nordisk, Bausch Health • Equity: Gila Therapeutics, Scientific Intake, Epitomee, ReDesign Health 2 05/06/2019 2 Objectives At the end of the session, attendees will be able to • identify when to start a weight loss medication, • identify how to choose the right one for the right patient, • identify when to combine approaches for better results and • discuss future prospects in obesity pharmacotherapy. • 3 Should we treat obesity with drugs? 4 05/06/2019 3 Should we treat obesity with drugs? No! not by themselves Yes! when patients need help • Drugs don’t work on their own. • For patients who struggle to lose They work through biology to enough weight to get health reinforce the patient’s intention to benefits, drugs can help them adhere to a dietary plan. Better better adhere to the dietary plan adherence = more weight loss. to lose more weight. Drugs also Drugs also sustain lost weight as sustain weight loss as long as they long as they are taken. are taken. 5 Rationale for Medications in Obesity Management • Food intake is biologically determined. • Weight loss is opposed and regain promoted by physiology of reduced obese state. • Medications work through biology of appetite regulation to help patients adhere to diet plans. -

Decrease of Fructosamine Levels During Treatment with Adalimumab
European Journal of Endocrinology (2007) 156 291–293 ISSN 0804-4643 CASE REPORT Decrease of fructosamine levels during treatment with adalimumab in patients with both diabetes and rheumatoid arthritis I C van Eijk1, M J L Peters1,2, M T Nurmohamed1,2,3, A W van Deutekom4, B A C Dijkmans1,2 and S Simsek4 1Department of Rheumatology, Jan van Breemen Institute, Amsterdam, The Netherlands, 2Department of Rheumatology, VU University Medical Center, Amsterdam, The Netherlands, 3Department of Internal Medicine, VU University Medical Center, Amsterdam, The Netherlands and 4Department of Endocrinology/Diabetes Center, VU University Medical Center, PO Box 7057, 1007 MB, Boelelaan 1117, Amsterdam, The Netherlands (Correspondence should be addressed to S Simsek; Email: [email protected]) Abstract Tumour necrosis factor a (TNFa) is a pro-inflammatory cytokine which has been closely linked to obesity and insulin resistance. We present two cases of patients with rheumatoid arthritis (RA) and concomitant diabetes mellitus, who showed a marked decrease of fructosamine levels after initiating therapy with adalimumab, a TNFa-blocking agent, for active RA. This finding may implicate that TNFa blockade causes better glycaemic control in RA patients with concomitant diabetes, possibly by improving insulin resistance. European Journal of Endocrinology 156 291–293 Introduction Research design and methods Tumour necrosis factor a (TNFa), a pro-inflammatory A patient with known diabetes type 1 and concomitant cytokine, plays an important role in inflammatory and RA showed a marked improvement of HbA1c levels after autoimmune diseases like rheumatoid arthritis (RA). initiation of adalimumab, a recombinant human IgG1- TNFa has also been closely linked to obesity and insulin MAB, therapy for active RA when she visited the resistance (1). -

Comparative Evaluation of Fructosamine and Hba1c As a Marker of Glycemic Control in Type 2 Diabetes: a Hospital Based Study
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Comparative Evaluation of Fructosamine and HbA1c as a Marker of Glycemic Control in Type 2 Diabetes: A Hospital Based Study Dr. Jyoti Goyal1, Dr. Nibhriti Das2, Dr. Navin Kumar3, Ms. Seema Raghav4, Dr. Paramjeet Singh Bhatia5, Dr. Karunesh Prasad Singh6, Dr. Sabari Das7 1DNB, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India- 281003, 2Ex-Director of Laboratory services and Additional Dean Research and Academics, Nayati Healthcare and Research Centre, Mathura, India- 281003, 3Ph.D, Biostatistitian, Department of Biostatistics, Nayati Healthcare and Research Centre, Mathura, India-281003. 4M.Sc., Certified Diabetes Educator, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 5MD, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 6MD Physician, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 7Department of Laboratory Medicine, Nayati Healthcare and Research Centre, Mathura, India- 281003, Corresponding Author: Dr. Jyoti Goyal ABSTRACT Introduction: Management of type 2 diabetes revolves around achievement of target glycemic control with the help of antidiabetic drugs or insulin. There are various markers for measurement of glyceamic control like HbA1c, Mean Blood Glucose and fructosamine levels. Though HbA1c is a well validated standard method for assessment of glycemic control but it has also got certain limitations. Fructosamine, a less explored method may be used as an alternative marker for an assessment of glycemic control in cases where HbA1c is unreliable or unavailable. The objective of this study is to compare the fructosamine levels with HbA1c in assessment of glycemic control in type 2 diabetics so as to assess the utility of fructosamine as an alternative marker for evaluation of glucose control. -

Acute Renal Failure in Patients with Type 1 Diabetes Mellitus G
Postgrad Med J: first published as 10.1136/pgmj.70.821.192 on 1 March 1994. Downloaded from Postgrad Med J (1994) 70, 192- 194 C) The Fellowship of Postgraduate Medicine, 1994 Acute renal failure in patients with type 1 diabetes mellitus G. Woodrow, A.M. Brownjohn and J.H. Turney Renal Unit, Leeds General Infirmary, Great George Street, Leeds LSJ 3EX, UK Summary: Acute renal failure (ARF) is a serious condition which still carries a mortality of around 50%. People with diabetes may be at increased risk of developing ARF, either as a complication of diabetic ketoacidosis or hyperosmolar coma, increased incidence of cardiovascular disease, or due to increased susceptibility ofthe kidney to adverse effects in the presence ofunderlying diabetic renal disease. During the period 1956-1992, 1,661 cases of ARF have been treated at Leeds General Infirmary. Of these, we have identified 26 patients also having type 1 diabetes. ARF due to diabetic ketoacidosis is surprisingly uncommon (14 cases out of 23 patients whose notes were reviewed). All cases of ARF complicating ketoacidosis in the last decade have been associated with particularly severe illness requiring intensive care unit support, rather than otherwise 'uncomplicated' ketoacidosis. We discuss the conditions that may result in ARF in patients with diabetes and the particular difficulties that may be encountered in management. Introduction People with diabetes may be at increased risk of Results developing acute renal failure (ARF). Acute pre- copyright. renal failure may occur as a result ofthe severe fluid Of 23 patients with type 1 diabetes complicated by depletion associated with diabetic ketoacidosis and ARF, diabetic ketoacidosis was the main underly- non-ketotic hyperosmolar coma. -

Metformin for Prediabetes
FACT SHEET FOR PATIENTS AND FAMILIES Metformin for Prediabetes What is metformin? Metformin [met-FORE-min] is a medication that is often prescribed for prediabetes in order to help prevent type 2 diabetes. It is taken by mouth (orally) as a pill. Like other prediabetes medications, it works best when you follow the rest of your treatment plan, like improving your nutrition by eating more fruits and vegetables, being physically active every day, and in most cases, losing weight. What does it do? Metformin helps lower your blood glucose (blood sugar). It does this by: Does metformin cause hypoglycemia • Decreasing the amount of glucose released by your (low blood glucose)? liver. Less glucose enters into your bloodstream. No. Metformin doesn’t cause hypoglycemia by itself. • Increasing the ability of your muscles to use glucose But combined with other medications, vigorous for energy. As more glucose is used, more glucose exercise, or too little food, it can make your blood leaves your bloodstream. glucose drop too low. Why is metformin important Since low blood glucose can be dangerous, make sure for my health? that you and your family know the symptoms. These include feeling shaky, sweaty, hungry, and irritable. Metformin can’t cure your prediabetes. But, by If you have these symptoms, take some quick-acting helping control your blood glucose, it can help sugar. Good sources are 3 or 4 glucose tablets, a half- prevent type 2 diabetes, especially among those at cup of fruit juice or regular soda, or a tablespoon of highest risk for developing type 2 diabetes. -

Effect of Biochemical Parameters Showing Atherogenicity in Type 2 Diabetic Nephropathy
International Journal of Basic and Applied Chemical Sciences ISSN: 2277-2073 (Online) An Online International Journal Available at http://www.cibtech.org/jcs.htm 2012 Vol. 2 (4) October-December pp.26-30/Raja and Shaker Research Article EFFECT OF BIOCHEMICAL PARAMETERS SHOWING ATHEROGENICITY IN TYPE 2 DIABETIC NEPHROPATHY *G. Raja and Ivvala Anand Shaker *Department of Biochemistry, Melmaruvathur Adhiparasakthi Institute of Medical Sciences and Research, Melmaruvathur-603319, Tamil Nadu, India *Author for Correspondence ABSTRACT Diabetic nephropathy is one of the major complications of diabetes mellitus characterized by frequent microalbuminuria, elevated arterial blood pressure, a persistent decline in glomerular filtration rate and a high risk of cardiovascular morbidity and mortality. The study comprised of 30 Diabetic mellitus (DM) with microalbuminuria patients (Group 3), 30 DM without microalbuminuria patients (group 2) compared with 30 healthy controls (Group 1). Fasting glucose, post prandial glucose, lipid profile, fructosamine and microalbuminuria were investigated in all the groups. The significant increase in serum fructosamine, fasting and post prandial glucose levels along with increased microalbuminuria observed in group 3 patients compared to group 2 and group 1 patients. Hyperglycemia, increased fructosamine and increased Cholesterol, triglycerides with decreased HDL-cholesterol levels indicates the major risk of atherogenicity. Key Words: Diabetic nephropathy, Microalbuminuria, Fructosamine and Atherogenicity INTRODUCTION Diabetes mellitus (DM) is a disease in which the hallmark feature is elevated blood glucose concentrations due to loss of insulin-producing pancreatic β-cells (type 1 diabetes) or through loss of insulin responsiveness in its target tissues (type 2 diabetes). Type 1 diabetes usually begins to manifest in childhood and early adulthood, but type 2 diabetes is typically a disease for which increased age is a risk factor (Schwarz, 2009). -

Yangxin Tongmai Formula Ameliorates Impaired Glucose Tolerance in Children with Graves’ Disease Through Upregulation of the Insulin Receptor Levels
Acta Pharmacologica Sinica (2018) 39: 923–929 © 2018 CPS and SIMM All rights reserved 1671-4083/18 www.nature.com/aps Article Yangxin Tongmai Formula ameliorates impaired glucose tolerance in children with Graves’ disease through upregulation of the insulin receptor levels Yan-hong LUO1, Min ZHU1, Dong-gang WANG1, Yu-sheng YANG1, Tao TAN2, Hua ZHU2, Jian-feng HE1, * 1Children’s Hospital Chongqing Medical University, Chongqing 400000, China; 2Department of Surgery, Davis Heart and Lung Research Institute, the Ohio State University Wexner Medical Center, Columbus, OH 43210, USA Abstract Graves’ disease (GD) is the leading cause of hyperthyroidism, and the majority of GD patients eventually develop disorders of glucose handling, which further affects their quality of life. Yangxin Tongmai formula (YTF) is modified from a famous formula of traditional Chinese medicine for the treatment of cardiovascular diseases. In this study we investigated the potential effects of YTF in the treatment of pediatric GD patients with impaired glucose tolerance. Forty pediatric GD patients and 20 healthy children were recruited for this clinical study. Based on the glucose tolerance, the GD patients were divided into two groups: 20 patients displayed impaired glucose tolerance, while the other 20 patients displayed normal glucose tolerance. YTF was orally administered for 60 days. YTF administration significantly ameliorated the abnormal glucose tolerance and insulin sensitivity in the GD patients with impaired glucose tolerance. To determine the molecular mechanisms of this observation, the number of plasma insulin receptors was determined by ELISA. Before treatment, the fasting and postprandial levels of the insulin receptor were significantly lower in patients with impaired glucose tolerance compared with those in patients with normal glucose tolerance and healthy children. -

Insulin Sensitivity and Resistin Levels in Gestational Diabetes Mellitus And
European Journal of Endocrinology (2008) 158 173–178 ISSN 0804-4643 CLINICAL STUDY Insulin sensitivity and resistin levels in gestational diabetes mellitus and after parturition Ana Megia, Joan Vendrell, Cristina Gutierrez, Modest Sabate´1, Montse Broch, Jose´-Manuel Ferna´ndez-Real2 and Inmaculada Simo´n Endocrinology and Diabetes Research Department, University Hospital of Tarragona ‘Joan XXIII’, ‘Pere Virgili’ Institute, ‘Rovira i Virgili’ University, 43007, Tarragona, Spain, 1Laboratory Department, Hospital ‘ St Pau i Sta. Tecla’, 43003, Tarragona, Spain and 2Endocrinology and Diabetes Unit, University Hospital ‘Josep Trueta’, 17007, Girona, Spain (Correspondence should be addressed to A Megia who is now at Seccio´ d’endocrinologı´a, Hospital Universitari ‘Joan XXIII’ de Tarragona, c/Mallafre´ Guasch, 4.43007 Tarragona, Spain; Email: [email protected]) Abstract Context: Resistin is expressed and secreted by the placenta during pregnancy. Increased serum resistin levels have been found in the second half of normal pregnancy, but its role in the pathogenesis of the insulin resistance of pregnancy is undetermined. Objective: The objective of the study was to assess the relationship between circulating resistin levels and insulin sensitivity in gestational diabetes mellitus (GDM). Design and setting: A case (nZ23)–control (nZ35) study was performed at the obstetrics and endocrinology clinic of a university hospital. Patients: In total, 58 Caucasian women with a singleton pregnancy who had been referred for a 100 g oral glucose tolerance test were enrolled between the weeks 26 and 30, and 22 women with GDM were also evaluated after pregnancy. Main outcome measures: Serum resistin and insulin sensitivity in GDM during and after pregnancy. The relationship of resistin to metabolic abnormalities was evaluated.