Federal Register/Vol. 85, No. 229/Friday
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MMWR, Volume 69, Issue 47 — November 27,2020
Morbidity and Mortality Weekly Report Weekly / Vol. 69 / No. 47 November 27, 2020 Sexual Violence in the Media: An Exploration of Traditional Print Media Reporting in the United States, 2014–2017 Olivia Egen, MPH1,2; Laura M. Mercer Kollar, PhD2; Jenny Dills, MPH2; Kathleen C. Basile, PhD2; Bethlehem Besrat, MPH3; Laura Palumbo, MA4; Kellie E. Carlyle, PhD5 Sexual violence is prevalent and, for many victims, begins early random sample of 2,600 articles from 48 of the top 50 tradi- in life (1). In the United States, one in five women and one in tional print media outlets distributed in the United States (8) 38 men report completed or attempted rape victimization during available via electronic newspaper databases.† Outlets were their lifetime, with 43.2% of female and 51.3% of male victims reporting that their first rape victimization occurred before age † Newspaper databases: News Bank Inc. (https://www.newsbank.com/); Gale OneFile (https://www.gale.com/databases/gale-onefile); US Newsstream 18 years (1). Media have been shown to act as a socializing agent (https://about.proquest.com/products-services/nationalsnews_shtml.html). for a range of health and social behaviors (2). Media portrayals might influence, reinforce, or modify how the public responds INSIDE to incidents of sexual violence and their support for prevention efforts and media might construct a lens through which the public 1762 Decline in SARS-CoV-2 Antibodies After Mild can understand who is affected by sexual violence, what forms it Infection Among Frontline Health Care Personnel takes, why it happens, and who is responsible for addressing it (3). -

Federal Register/Vol. 85, No. 229/Friday, November 27, 2020/Proposed Rules
76302 Federal Register / Vol. 85, No. 229 / Friday, November 27, 2020 / Proposed Rules DEPARTMENT OF COMMERCE required fields, and enter or attach your Background comments. We listed twenty coral species as National Oceanic and Atmospheric Instructions: You must submit threatened under the ESA effective Administration comments by the above to ensure that October 10, 2014 (79 FR 53851, we receive, document, and consider September 10, 2014). Five of the corals 50 CFR Parts 223 and 226 them. Comments sent by any other occur in the Caribbean: Orbicella [Docket No. 200918–0250] method or received after the end of the annularis, O. faveolata, O. franksi, comment period, may not be Dendrogyra cylindrus, and RIN 0648–BG26 considered. All comments received are Mycetophyllia ferox. The final listing a part of the public record and will determinations were all based on the Endangered and Threatened Species; generally be posted to http:// best scientific and commercial Critical Habitat for the Threatened www.regulations.gov without change. information available on a suite of Caribbean Corals All Personal Identifying Information (for demographic, spatial, and susceptibility example, name, address, etc.) components that influence the species’ AGENCY: National Marine Fisheries vulnerability to extinction in the face of Service (NMFS), National Oceanic and voluntarily submitted by the commenter continuing threats over the foreseeable Atmospheric Administration (NOAA), may be publicly accessible. Do not future. All of the species had undergone Commerce. submit Confidential Business Information or otherwise sensitive or population declines and are susceptible ACTION: Proposed rule; request for protected information. to multiple threats, including: Ocean comments. NMFS will accept anonymous warming, diseases, ocean acidification, ecological effects of fishing, and land- SUMMARY: We, NMFS, propose to comments (enter ‘‘N/A’’ in the required based sources of pollution. -

Early Dance Division Calendar 17-18
Early Dance Division 2017-2018 Session 1 September 9 – November 3 Monday Classes Tuesday Classes September 11 Class September 12 Class September 18 Class September 19 Class September 25 Class September 26 Class October 2 Class October 3 Class October 9 Class October 10 Class October 16 Class October 17 Class October 23 Class October 24 Class October 30 Last Class October 31 Last Class Wednesday Classes Thursday Classes September 13 Class September 14 Class September 20 Class September 21* Class September 27 Class September 28 Class October 4 Class October 5 Class October 11 Class October 12 Class October 18 Class October 19 Class October 25 Class October 26 Class November 1 Last Class November 2 Last Class Saturday Classes Sunday Classes September 9 Class September 10 Class September 16 Class September 17 Class September 23 Class September 24 Class September 30* Class October 1 Class October 7 Class October 8 Class October 14 Class October 15 Class October 21 Class October 22 Class October 28 Last Class October 29 Last Class *Absences due to the holiday will be granted an additional make-up class. Early Dance Division 2017-2018 Session 2 November 4 – January 22 Monday Classes Tuesday Classes November 6 Class November 7 Class November 13 Class November 14 Class November 20 No Class November 21 No Class November 27 Class November 28 Class December 4 Class December 5 Class December 11 Class December 12 Class December 18 Class December 19 Class December 25 No Class December 26 No Class January 1 No Class January 2 No Class January 8 Class -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -
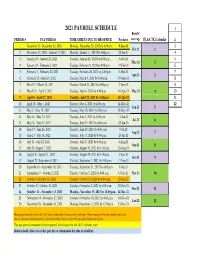
Payroll Calendar 2021
2021 PAYROLL SCHEDULE 1 Benefit PERIOD # PAY PERIOD TIME SHEETS DUE TO HR OFFICE Paydates coverage FLSA 7K Calendar 2 1 December 13- December 26, 2020 Monday, December 28, 2020 by 4:00 p.m. 8-Jan-21 3 Feb-21 1 2 December 27, 2020 - Janurary 9, 2021 Monday, January 11, 2021 by 4:00 p.m. 22-Jan-21 4 3 January 10 - January 23, 2021 Tuesday, January 26, 2021 by 4:00 p.m. 5-Feb-21 5 Mar-21 2 4 January 24 - February 6, 2021 Tuesday, February 9, 2021 by 4:00 p.m. 19-Feb-21 6 5 February 7 - February 20, 2021 Tuesday, February 26, 2021 by 4:00 p.m. 5-Mar-21 7 Apr-21 3 6 February 21 - March 6, 2021 Tuesday, March 9, 2021 by 4:00 p.m. 19-Mar-21 8 7 March 7 - March 20, 2021 Tuesday, March 23, 2021 by 4:00 p.m. 2-Apr-21 9 8 March 21 - April 3, 2021 Tuesday, April 6, 2021 by 4:00 p.m. 16-Apr-21 May-21 4 10 9 April 4 - April 17, 2021 Tuesday, April 20, 2021 by 4:00 p.m. 30-Apr-21 11 10 April 18 - May 1, 2021 Tuesday, May 4, 2021 by 4:00 p.m. 14-May-21 12 Jun-21 5 11 May 2 - May 15, 2021 Tuesday, May 18, 2021 by 4:00 p.m. 28-May-21 12 May 16 - May 29, 2021 Tuesday, June 1, 2021 by 4:00 p.m. 11-Jun-21 Jul-21 6 13 May 30 - June 12, 2021 Tuesday, June 15, 2021 by 4:00 p.m. -

Thanksgiving Weather Louisville, Kentucky
Thanksgiving Weather Louisville, Kentucky Highest temperature: 73° on November 26, 1896 Highest daily average temperature: 65.5° on November 26, 1896 Lowest temperature: 8° on November 27, 1930 Lowest average daily temperature: 14.5° on November 27, 1930 Wettest: 2.19” on November 25, 2010 Snowiest: 1.2” on November 24, 1938 Deepest Snow Cover: 1” on November 28, 1929; November 27, 1930; and November 25, 1971 High Temp Low Temp Precipitation Snowfall Snow Depth November 28, 1872 39 19 0 November 27, 1873 49 34 0 November 26, 1874 46 25 0 November 25, 1875 52 27 0.05 November 30, 1876 33 24 0.03 November 29, 1877 32 23 0 November 28, 1878 45 36 0 November 27, 1879 65 56 1 November 25, 1880 31 24 0.23 November 24, 1881 27 19 T November 30, 1882 42 32 0 November 29, 1883 56 37 0 November 27, 1884 60 45 0 0 November 26, 1885 49 38 0 0 November 25, 1886 39 25 T T November 24, 1887 60 48 0.32 0 November 29, 1888 42 37 0.01 0 November 28, 1889 37 23 0.06 0.1 November 27, 1890 51 27 0 0 November 26, 1891 52 37 T 0 November 24, 1892 38 16 0 0 November 30, 1893 49 38 0 0 November 29, 1894 40 23 T 0 November 28, 1895 55 28 0 0 November 26, 1896 73 58 0 0 November 25, 1897 56 47 0.23 0 November 24, 1898 35 21 0 0 November 30, 1899 66 48 0 0 November 29, 1900 39 31 0 0 0 High Temp Low Temp Precipitation Snowfall Snow Depth November 28, 1901 42 27 0 0 0 November 27, 1902 37 32 T T 0 November 26, 1903 29 19 0 0 0 November 24, 1904 66 35 0 0 0 November 30, 1905 30 22 0 0 0 November 29, 1906 43 32 0 0 0 November 28, 1907 51 36 0 0 0 November 26, 1908 -

76180 Federal Register / Vol
76180 Federal Register / Vol. 85, No. 229 / Friday, November 27, 2020 / Rules and Regulations DEPARTMENT OF HEALTH AND Mail Stop C4–26–05, 7500 Security analysis using the prescription drugs HUMAN SERVICES Boulevard, Baltimore, MD 21244–1850. and countries in the MFN Model For information on viewing public suggests Medicare Part B paid at least Centers for Medicare & Medicaid comments, see the beginning of the 2.05 times as much as other higher- Services SUPPLEMENTARY INFORMATION section. income countries in 2018.3 The Centers FOR FURTHER INFORMATION CONTACT: for Medicare & Medicaid Services’ 42 CFR Part 513 Andrew York, 410–786–7400. (CMS) Center for Medicare and [CMS–5528–IFC] SUPPLEMENTARY INFORMATION: Medicaid Innovation (Innovation Inspection of Public Comments: All Center) is taking action on President RIN 0938–AT91 comments received before the close of Trump’s goal to lower drug costs and seeking to realign financial incentives Most Favored Nation (MFN) Model the comment period are available for viewing by the public, including any by implementing the Most Favored AGENCY: Centers for Medicare & personally identifiable or confidential Nation (MFN) Model as described in Medicaid Services (CMS), HHS. business information that is included in this IFC. Medicare pays substantially more ACTION: Interim final rule with comment a comment. We post all comments than other countries for many of the period. received before the close of the highest-cost Medicare Part B drugs that comment period on the following beneficiaries receive in an outpatient SUMMARY: This interim final rule with website as soon as possible after they setting for which Medicare Part B allows comment period (IFC) implements the have been received: http:// separate payment.4 In many instances, Most Favored Nation (MFN) Model, a www.regulations.gov. -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -

Daily COVID-19 Update for November 27, 2020
Daily COVID-19 Update for November 27, 2020: TOTAL – 83 Thursday, November 26, 2020: Cumberland County Department of Health – 23 Deaths – 1 Female 80s Bridgeton Male 60s Bridgeton Female 30s Bridgeton Female 10s Bridgeton Female 20s Bridgeton Male 60s Bridgeton Female 10s Bridgeton Male 30s Bridgeton Female 10s Bridgeton Male 40s Bridgeton Female 20s Fairfield Male 90s Hopewell Male 10s Hopewell Female 60s Hopewell Male 20s Hopewell Female 70s Hopewell Male 40s Hopewell Female 50s Hopewell Female 40s Millville Female 60s Millville Male 20s Millville Female 20s Upper Deerfield Male 70s Upper Deerfield Deaths Female 90s Hopewell Vineland Health Department – 19 Deaths – 1 Female 20s Vineland Female 50s Vineland Female 30s Vineland Female 90s Vineland Female 50s Vineland Female 10s Vineland Female 60s Vineland Male 70s Vineland Female 40s Vineland Male 10s Vineland Male 60s Vineland Female 40s Vineland Female 40s Vineland Male 70s Vineland Male 70s Vineland Male 60s Vineland Female 40s Vineland Female 0-9 Vineland Male 10s Vineland Deaths Female 70s Vineland Friday, November 27, 2020: Cumberland County Department of Health – 30 Deaths – 1 Male 50s Bridgeton Male 60s Bridgeton Female 20s Bridgeton Male 40s Bridgeton Male 40s Bridgeton Female 20s Bridgeton Male 60s Fairfield Male 50s Fairfield Male 40s Fairfield Female 60s Fairfield Male 20s Fairfield Female 20s Fairfield Female 80s Hopewell Female 40s Hopewell Female 60s Lawrence Male 70s Millville Female 30s Millville Male 70s Millville Female 60s Millville Male 40s Millville Female 60s Millville -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -

Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January -

Due Date Chart 201803281304173331.Xlsx
Special Event Permit Application Due Date Chart for Events from January 1, 2019 - June 30, 2020 If due date lands on a Saturday or Sunday, the due date is moved to the next business day Event Date 30 Calendar days 90 Calendar Days Tuesday, January 01, 2019 Sunday, December 02, 2018 Wednesday, October 03, 2018 Wednesday, January 02, 2019 Monday, December 03, 2018 Thursday, October 04, 2018 Thursday, January 03, 2019 Tuesday, December 04, 2018 Friday, October 05, 2018 Friday, January 04, 2019 Wednesday, December 05, 2018 Saturday, October 06, 2018 Saturday, January 05, 2019 Thursday, December 06, 2018 Sunday, October 07, 2018 Sunday, January 06, 2019 Friday, December 07, 2018 Monday, October 08, 2018 Monday, January 07, 2019 Saturday, December 08, 2018 Tuesday, October 09, 2018 Tuesday, January 08, 2019 Sunday, December 09, 2018 Wednesday, October 10, 2018 Wednesday, January 09, 2019 Monday, December 10, 2018 Thursday, October 11, 2018 Thursday, January 10, 2019 Tuesday, December 11, 2018 Friday, October 12, 2018 Friday, January 11, 2019 Wednesday, December 12, 2018 Saturday, October 13, 2018 Saturday, January 12, 2019 Thursday, December 13, 2018 Sunday, October 14, 2018 Sunday, January 13, 2019 Friday, December 14, 2018 Monday, October 15, 2018 Monday, January 14, 2019 Saturday, December 15, 2018 Tuesday, October 16, 2018 2019 Tuesday, January 15, 2019 Sunday, December 16, 2018 Wednesday, October 17, 2018 Wednesday, January 16, 2019 Monday, December 17, 2018 Thursday, October 18, 2018 Thursday, January 17, 2019 Tuesday, December 18, 2018