January 7, 2021 To: Cargill Customers Re: Safe Feed for Animals Letter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Navigator Newsletter August 2016
Navigator Newsletter August 2016 Food & Dietary Supplies The Experienced Leader in Group Purchasing • Produce Alliance > A monthly newsletter to keep you informed. • Smucker's > Thank you for being a valued member of Navigator Group Purchasing, Inc. We are the experienced leader in healthcare and hospitality purchasing services offering you Full Transparency Reporting, Vendor Flexibility, and Realized Savings. Medical Products & Services Navigator is your resource for spend management. • ConvaTec > We are here to help you maximize savings • United Lab > opportunities and choice with the industry's top vendors and manufacturers. Our goal is to keep you informed on contract updates and promotions from our preferred vendor partners as well as industry news. Business Products & Services Please contact your Navigator • Phoenix Textile > Account Representative for more information. • Sherwin-Williams > Visit Our Website • Shred-it > Find out how a Navigator membership can work for you. Call us today! 1-800-642-3020 Industry News • Foodbuy Reports: - Member Advantage > - Pricewatch > - Produce Market Advisory > - Inflation Q2 • Upcoming Events PA SERVICES FOOD SAFETY & QUALITY ASSURANCE CENTRAL PROCUREMENT SERVICES At Produce Alliance, food safety and Quality assurance is not Through our 4+ billion network our dedicated & just our management initiative, it is our way of life. From the experienced procurement arm manages price, grower farm to your customer’s plate, our dedication to food safety is contract compliance, Quality & logistics daily. -

Cargill Premix & Nutrition: Transforming Talent Management
Cargill Premix & Nutrition: Transforming Talent Management Michael Gunderson Associate Professor and Associate Director of Research Center for Food and Agricultural Business, Purdue University Wes Davis Graduate Assistant Center for Food and Agricultural Business, Purdue University This case was prepared by Michael Gunderson, associate professor and associate director of research, and Wes Davis, graduate assistant, Center for Food and Agricultural Business, Purdue University. The authors would like to thank Cargill Premix & Nutrition, particularly Heather Imel, vice president of human capital, Cargill Premix & Nutrition North America. The case is a basis for class discussion and represents the views of the authors, not Purdue University. No part of this publication may be reproduced or transmitted in any form without written permission from Purdue University. 2 | Cargill Premix & Nutrition: Transforming Talent Management © 2017 Purdue Univerisity In early 2016 Charles Shininger, managing director for Cargill Premix & Nutrition North America, takes part in a leadership meeting with other business unit managers and platform leaders at Cargill’s global headquarters in Minneapolis. Having just completed a successful quarter, he looks forward to reviewing the global enterprise’s performance and discussing business plans for the year. As the meeting begins, the global executive team of nine corporate officers discusses overall performance. Cargill has experienced declining annual operating earnings since 2011 (Exhibit 1). Some business units have performed well; however, changing customer preferences, political and regulatory uncertainty, macroeconomic challenges, and reinvented customer business models have eroded overall enterprise earnings and led the global executive team to consider what changes are necessary to reclaim performance. Exhibit 1: Over the last six years, operating earnings have been suppressed compared to FY 2011. -

Category Slaughterhouse (For Meat and Poultry) / Breaking Location
Supply Chain Disclosure Poultry Upstream Snapshot: December 2018 Published: March 2019 Category Slaughterhouse (for meat and poultry) / Breaking location (for eggs) Location Address Country Chicken Abatedouro Frigorifico Avenida Antonio Ortega nº 3604, Bairro Pinhal , Cabreuva – São Paulo – Brasil Brazil Chicken Agrosul Agroavícola Industrial S.A. Rua Waldomiro Freiberger, 1000 - São Sebastião do Caí, Rio Grande do Sul, Brasil Brazil Turkey Agrosuper Chile Condell Sur 411, Quilpué, Valparaiso, Región de Valparaíso, Chile Chile Chicken Agrosuper LTD Camino La Estrella 401, Rancgua, Chile Chile Poultry Animex Foods Sp. z o.o. Sp. k. Morliny Animex Foods Sp. z o.o. Sp. k. Morliny 15, 14-100 Ostróda, Branch of Iława, Poland Poland Chicken Belwood Lowmoor Business Park Kirkby-In-Ashfield, Nottingham NG17 7ER UK Turkey Biegi Foods GmbH Schaberweg 28 61348 Bad Homburg Germany Poultry BODIN LES TERRES DOUCES SAINTE-HERMINE 85210 France Poultry Bodin et Fils ZA Les Terres Douces, Sainte Hermine, France France Chicken BOSCHER VOLAILLES ZA de Guergadic 22530 Mûr de Bretagne France Chicken Boxing County Economic Development Zone Xinsheng Food Co., Ltd. Fuyuan two road, Boxing County Economic Development Zone, Binzhou China Duck Burgaud Parc Activ De La Bloire 42 Rue Gustave Eiffel 85300 France Turkey Butterball - Carthage 411 N Main Street, Carthage, MO 64836 USA Turkey Butterball - Mt. Olive 1628 Garner's Chapel Road, Mt Olive, NC 28365 USA Chicken C Vale - Paloina Av Ariosvaldo Bittencourt, 2000 - Centro - Palotina, PR Brazil Duck Canards d'Auzan -

Cargill Non-GMO Solutions
Cargill Non-GMO Solutions Cargill helps customers grow and protect their brands, and reduce time-to-market Non-GMO* is one of the Food category growth rates fastest growing claims in % growth of $ sales over prior year the U.S. food industry.1 12% 11% 11% A recent Cargill study showed that GMO is top 10% of mind when consumers are asked what they avoid when purchasing food. For over 15 years Cargill has helped customers navigate supply-chain challenges, source non-GMO ingredients, and grow their 2% non-GMO business. 1% 1% 1% 2012 2013 2014 2015 From dedicated producer programs to the industry’s broadest ingredient Total (+1% CAGR) portfolio, Cargill is the right Non-GMO Organic Natural (+11% CAGR) partner to help food and beverage manufacturers grow and protect Source: White Wave at 2016 CAGNY Conference their brands by delivering non-GMO products to consumers. 1 Source: Mintel, March 2016 Cargill.com/food-beverage Cargill markets the industry’s broadest portfolio of non-GMO ingredients. From sweeteners, starches and texturizers to oils, cocoa and chocolate, Cargill delivers the scale that food manufacturers need to get to market quickly and meet growing consumer demand. We offer a growing number of Non-GMO Project Verified ingredients so customers can feature America’s most recognizable non-GMO claim on their labels. Well-established producer programs for corn, soybeans and high oleic canola means unsurpassed supply chain assurance for our customers. Limited supply of non-GMO corn, soybeans and high oleic canola creates challenges for food and beverage companies seeking to scale production and meet growing consumer demand. -

Cargill and Technoserve Partnering to Support Rural Development Around the World
Cargill and TechnoServe Partnering to support rural development around the world Cargill has been working with TechnoServe since 2000, providing $6.6 million to build farmer capacity and improve the livelihoods of agricultural communities. In addition to funding, Cargill provides technical expertise to the programs, which have supported farmers and enterprises across Latin America, Africa and Asia. Our work together includes: Helping farmers improve their agricultural practices and increase access to inputs and markets Developing management skills among leaders of farmer cooperatives Providing technical and business expertise to small and medium food processors and enterprises Building a strong partnership for farmers In 2014, Cargill and TechnoServe in Nicaragua joined up to project has exceeded the targeted number of farmers develop IMPULSOR (Spanish for “driver or booster”), a four-year participating in the training and average costs for the farmers initiative to provide improved inputs, technical assistance and have reduced by 10 percent, despite recent drought conditions market access to 440 farmers that produce around half of the and instability in the country. Cargill and TechnoServe are in country’s sorghum harvest. A cereal grain, sorghum is widely discussions to determine the best way to sustain this impact. used in Central America as an alternative to maize; it responds better to drought and is used for feeding poultry and, in some cases, as an ingredient in tortillas and other staples. To date the Improving livelihoods and International Finance Corporation, is an industry-first cooperative empowering communities management program. Endorsed by the government, it combines 28 days of intensive classroom training with a year of Cargill and TechnoServe partner on a four-year program in India’s personalized on-the-ground coaching. -

Hugh Cargill Farm Management Plan
LAND USE and MANAGEMENT PLAN for the Hugh Cargill Farm Walden Street and Thoreau Street Assessors' Map H10; Parcels 0217, 0220, 0221, 0222 28+/- acres Goals and objectives The goals of this Management Plan are to provide guidance for the management of the remaining land of the Hugh Cargill Farm for its traditional uses, and recommend an integrated plan for management of previously developed parcels (excluding residentially developed areas) together with the remaining Hugh Cargill Farm. Traditional uses include: Drinking water protection and production, agriculture, benefit of the poor in Concord, wildlife protection, maintenance of a scenic gateway into Concord, and the general enjoyment of Concord’s citizens and school children. Open landscapes are declining regionally and in Concord, and this Plan is intended to ensure that the Hugh Cargill Farm land remain open by regular mowing or agricultural haying. The Cargill land provides a rural landscape on an important gateway into town, as well as a connection with the Hapgood Wright Town Forest. Invasive plant species should be selectively removed over time in order to restore the biodiversity of this site. These activities will be conducted in a way that maximizes the wildlife habitat provided by grasslands for butterflies, small mammals, and grassland nesting birds. Informal playing fields are also an accepted use under this plan. No activity may be conducted that will adversely impact the water quality of the Hugh Cargill well. Although some of the original land has been carved off for other uses (and acquisition funds placed in the trust to be disbursed to needy residents of Concord), this Plan seeks to integrate management of all lands, regardless of ownership, within the original bounds to be managed for the goals and objectives stated above. -

Cargill Inc. V. WDS Inc
UNITED STATES DISTRICT COURT WESTERN DISTRICT OF NORTH CAROLINA CHARLOTTE DIVISION DOCKET NO. 3:16-cv-00848-FDW-DSC CARGILL, INC., and CARGILL MEAT ) SOLUTIONS, CORP., ) ) Plaintiffs, ) ) vs. ) ORDER ) WDS, INC., JENNIFER MAIER, and ) BRIAN EWERT, ) ) Defendants. ) ) THIS MATTER is before the Court upon the filing of several post-trial motions by Plaintiffs and Defendants and one pending pre-trial motion filed by Plaintiffs. Before trial, Plaintiffs sought default judgment against all Defendants as sanction for abusive litigation practices. (Doc. No. 187). After trial, Plaintiffs filed a Motion for Award of Prejudgment Interest (Doc. No. 320), a Motion for Award of Attorneys’ Fees and Costs (Doc. No. 325), and a Memorandum of Law on the Unfair and Deceptive Trade Practice Act (Doc. No. 329).1 Defendants WDS, Inc. (“WDS”) and Brian Ewert (“Ewert”) move under Rule 50(b) and Rule 59 of the Federal Rules of Civil Procedure for judgment as a matter of law in its favor or in the alternative a new trial. (Doc. No. 322). Defendant Jennifer Maier (“Maier”) also filed a motion seeking judgment as a matter of law in her favor under Rule 50(b) or in the alternative, a new trial 1 Defendant Jennifer Maier filed a voluntary petition under Chapter 7 of the United States Bankruptcy Code, but the automatic stay has been terminated and modified to allow this case to “proceed in all respects to completion.” (See Doc. No. 346-1). Therefore, the Court can proceed on all motions and matters against all Defendants. (See Doc. Nos. 346, 348). 1 Case 3:16-cv-00848-FDW-DSC Document 366 Filed 03/28/18 Page 1 of 47 or amendment to the judgment under Rule 59. -

Meeting Record Handout
PERD U E (non-"enhanced") B\.S t-: U :~:•. :)) -: '! 1'= S,T E ''-i), Amount'" 110 LG '8 R E ("enhanced") BEST TO US€ OR I"/IUZE BY '\ MAR 2507 7OMoo P-171 American Heart Association ,.% !AeerJ A.meric.Jn "*11 ~ bod .:r leriatl , saltaledfa l anll~ Og 0% .,....1I\'_""',get Iron 4% ottallll'-lfat.....fat. Not.~centlOun:e ~ fiber,"', villmlllA. vllamln c. and Clllclulll. "hIt*It DeItf var_..bUIld01\'2.000 cabtI Hatura'· Chlde.n 8roth IngnHIlenta: ChIcken- Broth. Sa/t, Carrageenan. GLUTEN FREE 'STRIBUTED BY: PILGRIM'S PRIDE CORPORATION PO BOX 93 PJn88URG,TX 7: ... -I I "I ~-- \ ~ ;; \ ~ z -'=- ~ T !lflf~~IJlniWt ................. • $3.52 1 ca......110 Call OfT 'OJ" $1. 9 ,-..J'at2.5g SaturaTed Fat 0.5g TflltS Fatog Pof~nsaturated Fat Og Monounsaturated ~1;_ ':'2t .........,,~. I lail.,8Omg Rlghr Sfore. Rlahr Price f.I~_ ' Og Dietary Fiber Og Ameetcan ,..., $ 'dan SugarsOg" ....._tr__...& IIIPIIIIIaIIp lIroteln-23g 4ft; • ==...-'=-~=:..~==::----..,-"'~ 'lC) AiMCJAt~1H;.Io!y1lQ:SS!o ,.pawb'=:............._.. "IIIliIIl __1'IDIIr1l£1.If OIQlOe"OICIIH VItamIn A0% ~ c.iCiumO% 'PIIn:idtlilfi~ 0IlI0IIe dI8L OIMIIUTBIaY: MOlt =: AI rtJ .....---•......SI"UBIIaF. - .-[..111"\. GiIiII i" ~- "'-.I!i&. ' . What People Are Saying About "Pumped-Up" Poultry: "We want consumers to know what they're getting, said California Poultry Federation President Bill Mattos. We think it's kind of a fraud to sell something in the refrigerated (not frozen) case that looks fresh and feels fresh but contains up to 15 percent water and salt." The Seattle Times September 15, 2004 "Some poultry producers are adding a solution of sodium and water to raw poultry products in order to enhance flavor or increase moisture content. -

Cocoa and Chocolate Contents Language Table of Contents
Language Sustainability Progress Report 2018 - 2019 Cocoa and Chocolate Contents Language Table of Contents Published May 2020 Introduction Community Consumer Responsible 3 President’s welcome Wellbeing Confidence Business 4 Our approach 12 Progress at a glance 18 Progress at a glance 24 Operating in a safe, responsible and 13 Spotlight: A localized 19 Spotlight: Connecting 5 Taking cocoa to sustainable way the cloud approach to tackle child data to measure and labor in Indonesia report on supply chain 7 Impact at a glance sustainability Farmer Protect Transformation, About Livelihoods our Planet together 26 Cargill 9 Progress at a glance 15 Progress at a glance 21 Progress at a glance 28 Cocoa and Chocolate 10 Spotlight: Beyond 16 Spotlight: From 22 Spotlight: Coop 29 This report cocoa: taking a holistic satellites to farmers: a Academy 2.0: look at farmer resilience multi-layered approach Strengthening more against deforestation cooperatives with management tools Cargill | Cocoa and Chocolate | 2018-2019 Sustainability Progress Report 2 Introduction Language Dear stakeholders The world is faced with extraordinary chal- Digital technology enables us to provide our customers Transparency is imperative and we are committed to con- lenges. The ongoing COVID-19 pandemic is with fast and transparent sustainability data, helping them tinuously improve and refine how we show the progress measure and report the impact they achieve through the we make. This report focuses on progress both towards testing our resilience as a global community. Cargill Cocoa Promise. As of this year, half of the cocoa our goals and against the data of the previous year; it high- This reminds us how interconnected our world in our global direct supply chain is traceable from farm to lights many great accomplishments. -

Get High on the Wow Factor Page 24 Spring 2015
FOOD FANATICS FOOD FOOD PEOPLE MONEY & SENSE PLUS Regional Chinese Group Dining Fear of Failure I’ll Drink to That! The latest riffs revealed, Cash in on large parties, 7 nightmare busters, Gin is in, page 8 page 38 page 52 page 62 THE WOW FACTOR THE WOW Sharing the Love of Food—Inspiring Business Success SPRING 2015 BLOWN AWAY GET HIGH ON THE WOW FACTOR PAGE 24 SPRING 2015 FOOD Real Chinese Steps Out 8 America’s regional Chinese cuisine gets ADVERTISEMENT back to its roots. In the Raw 14 Tartare goes beyond beef, capers and PAGE 112 egg yolk. Tapping Into Maple Syrup 20 This natural sweetener breaks out of its morning routine. COVER STORY The Wow Factor 24 When the ordinary becomes extraordinary. MAPLE FOOD PEOPLE SYRUP GOES BOTH Bigger Is Better 38 Master a group mentality to cash in on WAYS— large parties. SWEET AND SAVORY Talk Shop PAGE 20 40 Upping the minimum wage: thumbs up or thumbs down? Road Trip to Las Vegas 44 Take a gamble on a restaurant off the strip. PREMIUM QUALITY SIGNATURE TASTE EXCEPTIONAL PERFORMANCE Download the app on iTunes or view the MONEY & SENSE magazine online at FOODFANATICS.COM The Secret to the Upsell 48 A seasoned dining critic says to ditch selling and focus on service. Nightmare Busters 52 Ways to combat 7 of the most common restaurant fears. I’ll Drink to That 62 Gin for the win: The original flavored spirit paves the way for focused beverage programs. WHEN THE TUNA IN TARTARE BECOMES A SNOOZER, GIVE OTHERS A TRY IN EVERY ISSUE (HINT: SALMON) PAGE 14 FOOD Trend Tracker 31 What’s turning up the heat and what’s cooling off. -

Industrial Agriculture, Livestock Farming and Climate Change
Industrial Agriculture, Livestock Farming and Climate Change Global Social, Cultural, Ecological, and Ethical Impacts of an Unsustainable Industry Prepared by Brighter Green and the Global Forest Coalition (GFC) with inputs from Biofuelwatch Photo: Brighter Green 1. Modern Livestock Production: Factory Farming and Climate Change For many, the image of a farmer tending his or her crops and cattle, with a backdrop of rolling fields and a weathered but sturdy barn in the distance, is still what comes to mind when considering a question that is not asked nearly as often as it should be: Where does our food come from? However, this picture can no longer be relied upon to depict the modern, industrial food system, which has already dominated food production in the Global North, and is expanding in the Global South as well. Due to the corporate take-over of food production, the small farmer running a family farm is rapidly giving way to the large-scale, factory farm model. This is particularly prevalent in the livestock industry, where thousands, sometimes millions, of animals are raised in inhumane, unsanitary conditions. These operations, along with the resources needed to grow the grain and oil meals (principally soybeans and 1 corn) to feed these animals place intense pressure on the environment. This is affecting some of the world’s most vulnerable ecosystems and human communities. The burdens created by the spread of industrialized animal agriculture are wide and varied—crossing ecological, social, and ethical spheres. These are compounded by a lack of public awareness and policy makers’ resistance to seek sustainable solutions, particularly given the influence of the global corporations that are steadily exerting greater control over the world’s food systems and what ends up on people’s plates. -
Cadenza Document
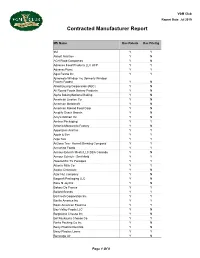
VGM Club Report Date: Jul 2019 Contracted Manufacturer Report Mfr Name Has Rebate Has Pricing 3M Y Y Abbott Nutrition Y N ACH Food Companies Y N Advance Food Products LLC AFP Y Y AdvancePierre Y Y Agro Farma Inc Y Y Ajinomoto Windsor Inc (formerly Windsor Frozen Foods) Y N Allied Buying Corporation (ABC) Y N All Round Foods Bakery Products Y N Alpha Baking/National Baking Y N American Licorice Co Y N American Metalcraft Y N American Roland Food Corp Y N Amplify Snack Brands Y N Amy's Kitchen Inc Y N Anchor Packaging Y Y Antonio Mozzarella Factory Y N Appetizers And Inc Y Y Apple & Eve Y Y Argo Tea Y Y Arizona Tea - Hornell Brewing Company Y Y Armanino Foods Y Y Armour-Eckrich Meats LLC DBA Carando Y N Armour Eckrich - Smithfield Y Y Ateeco/Mrs T's Pierogies Y Y Atlantic Mills Co Y Y Awake Chocolate Y N Azar Nut Company Y N Bagcraft Packaging LLC Y N Bake N Joy Inc Y N Bakery De France Y Y Ballard Brands Y Y BarFresh Corporation Inc Y Y Barilla America Inc Y Y Basic American Food Co Y Y Bay Valley Foods LLC Y N Belgioioso Cheese Inc Y N Bel Kaukauna Cheese Co Y Y Berks Packing Co Inc Y N Berry Plastics Diet Kits Y N Berry Plastics Liners Y Y Beverage Air Y N Page 1 Of 9 VGM Club Report Date: Jul 2019 Contracted Manufacturer Report Mfr Name Has Rebate Has Pricing Beyond Meat Y Y B&G Foods Inc Y Y Big City Reds /American Foods Y N Big Red Inc Y Y BioSelect N Y Biscomerica Corp.