Diffuse Cauda Equina Nerve Root Enhancement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acute Cauda Equina Syndrome Following Orthopedic Procedures As a Result of Epidural Anesthesia Lisa B
OPEN ACCESS Editor: Nancy E. Epstein, MD SNI: Spine For entire Editorial Board visit : Winthrop Hospital, Mineola, http://www.surgicalneurologyint.com NY, USA Case Report Acute cauda equina syndrome following orthopedic procedures as a result of epidural anesthesia Lisa B. E. Shields1, Vasudeva G. Iyer2, Yi Ping Zhang1, Christopher B. Shields1,3 1Norton Neuroscience Institute, Norton Healthcare, 2Neurodiagnostic Center of Louisville, 3Department of Neurological Surgery, University of Louisville School of Medicine, Louisville, Kentucky, USA E‑mail: Lisa B. E. Shields ‑ [email protected]; Vasudeva G. Iyer ‑ [email protected]; Yi Ping Zhang ‑ [email protected]; *Christopher B. Shields ‑ [email protected] *Corresponding author Received: 29 December 17 Accepted: 15 January 18 Published: 10 April 18 Abstract Background: Cauda equina syndrome (CES) is a rare complication of spinal or epidural anesthesia. It is attributed to direct mechanical injury to the spinal roots of the cauda equina that may result in saddle anesthesia and paraplegia with bowel and bladder dysfunction. Case Description: The first patient underwent a hip replacement and received 5 mL of 1% lidocaine epidural anesthesia. Postoperatively, when the patient developed an acute CES, the lumbar magnetic resonance imaging (MRI) scan demonstrated clumping/posterior displacement of nerve roots of the cauda equina consistent with adhesive arachnoiditis attributed to the patient’s previous L4‑L5 lumbar decompression/ fusion. The second patient underwent spinal anesthesia (injection of 10 mg of isobaric Access this article online bupivacaine for an epidural block) for a total knee replacement. When the patient Website: www.surgicalneurologyint.com developed an acute CES following surgery, the lumbar MRI scan showed an abnormal DOI: T2 signal in the conus and lower thoracic spinal cord over 4.3 cm. -

A Cauda Equina Syndrome in a Patient Treated with Oral Anticoagulants
Paraplegia 32 (1994) 277-280 © 1994 International Medical Society of Paraplegia A cauda equina syndrome in a patient treated with oral anticoagulants. Case report l l l 2 l J Willems MD, A Anne MD, P Herregods MD, R Klaes MD, R Chappel MD 1 Department of Physical Medicine and Rehabilitation, 2 Department of Neurosurgery, A.z. Middelheim, Lindendreef 1, B-2020 Antwerp, Belgium. The authors report a patient who was on oral anticoagulants because of mitral valve disease and who developed paraplegia from subarachnoid bleeding involv ing the cauda equina. The differential diagnosis, investigations and treatment of the cauda equina syndrome are described. Keywords: cauda equina syndrome; anticoagulants; subarachnoid haemorrhage; mitral valve disease. Case report A 32 year old woman from Chile presented with a complete paraplegia. She claimed that the paraplegia had developed progressively over 8 months. Initially she had paraesthesiae in her feet, followed by progressive paresis of both legs, beginning distally, over a period of 3 months. Two months after the onset of illness she complained of bladder incontinence. There was no history of trauma or low back pain. Clinical examination in our hospital revealed a flaccid paraplegia at L1 level, and loss of sensation from the groins to the feet, including saddle anaesthesia. The knee and ankle jerks were absent. The anal sphincter was atonic. She had an indwelling urethral catheter, and she was faecally incontinent. Myelography and a CT scan were carried out, and a space-occupying lesion at the level of T12-L4 (Figs 1, 2) was defined. Surgical ex ploration was done to determine the cause. -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

MR of Cranial and Spinal Meningeal Carcinomatosis: Comparison with CT and Myelography
709 MR of Cranial and Spinal Meningeal Carcinomatosis: Comparison with CT and Myelography George Krol 1 Thirty-nine patients with histologically proved primary neoplasms, focal neurologic Gordon Sze deficits, and positive CSF cytology were evaluated by enhanced cranial CT and MR, or Mark Malkin complete myelography and MR of the spine. Intracranial abnormalities were noted on Russell Walker CT in 56% of cases and included abnormal enhancement of subarachnoid space and ventricular walls, ventricular dilatation, obliteration of cortical sulci, and enhancing nodules within the subarachnoid cisterns and lumen of the lateral ventricles. Although the degree of ventricular enlargement and intraventricular tumor deposits were equally well seen on CT and MR, involvement of ventricular walls, tentorium, subarachnoid cisterns, or subarachnoid space interpreted as abnormal enhancement on CT was not readily appreciated on routine n- and T2-weighted spin-echo sequences. Forty-four percent of CT and 65% of MR studies were interpreted as normal. There was high correlation of myelographic findings with clinical diagnosis, and no false-negative myelograms. Nodular filling defects within the subarachnoid space, thickening and crowding of roots of the cauda equina, irregularity of individual roots, and scalloping of the subarachnoid membranes were demonstrated. MR was rather insensitive in detecting these changes, revealing a definite abnormality of the subarach noid space in 27% of patients with positive myelograms. False-negative interpretation of MR of the spine was made in 44% of cases. Neoplastic involvement of the leptomeningeal membranes may occur as a complication of neoplasms arising within the CNS or as a metastatic process originating from a distant primary tumor. -

Progressive Cauda Equina Syndrome and Extensive Calification/Ossification of the Lumbosacral Meninges
Ann Rheum Dis: first published as 10.1136/ard.44.4.277 on 1 April 1985. Downloaded from Annals of the Rheumatic Diseases, 1985, 44, 277-280 Case report Progressive cauda equina syndrome and extensive calification/ossification of the lumbosacral meninges J ROTES-QUEROL,' E TOLOSA,2 R ROSELLO,' AND J GRANADOS3 From the 1 Rheumatology Service, and the 2Neurology Service, Hospital Clinico y Provincial, Facultad de Medicina, C/ Casanova 143, Barcelona 08036, Spain and the 3Rheumatology Service, Hospital de la Mutua de Tarrasa, Tarrasa, (Provincia de Barcelona), Spain SUMMARY A patient with longstanding ankylosing spondylitis (AS) developed a cauda equina syndrome. The myelogram showed a block at the L2 level. Vertebral computerised tomography showed calcification in the centre of the spinal canal. The patient also had features suggestive of a diffuse idiopathic skeletal hyperostosis (DISH). Meningeal calcification has never been reported in AS, so we suggest that this is related to an associated DISH. Cauda equina syndrome has not been described in DISH, and calcification of meninges has not been reported in AS, so we suggest that the meningeal calcifications and associated cauda equina syndrome are related to DISH. copyright. Key words: diffuse idiopathic skeletal hyperostosis, ankylosing spondylitis, meningeal calcification. The appearance of a cauda equina syndrome in Case report patients with ankylosing spondylitis (AS) is well http://ard.bmj.com/ known, and nearly 30 cases of this association have been published, most of them recorded by The patient was a 63-year-old female. In 1977 when myelography or computerised tomography (CT). 1-10 she was 57 she consulted our Rheumatology Clinic Posterior saccular diverticula are seen when because of back pain and stiffness that had lasted for myelography is performed in the supine position. -
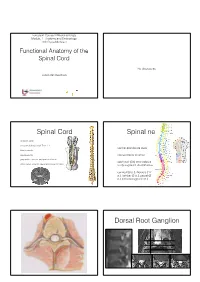
Functional Anatomy of the Spinal Cord
European Course in Neuroradiology Module 1 - Anatomy and Embryology 16th Cycle Module 1 Functional Anatomy of the Spinal Cord No disclosures Johan Van Goethem Spinal Cord Spinal nerves • vertebral canal • conus medullaris: adult Th12- L1 • ventral and dorsal roots • filum terminale • cauda equina • intervertebral foramen • gray matter: anterior and posterior horns • each pair (31) innervates a • white matter: anterior, lateral and posterior tracts body segment: dermatomes • cervical (8 p.), thoracic (12 p.), lumbar (5 p.), sacral (5 p.) and coccygeal (1 p.) Dorsal Root Ganglion Dorsal Root Ganglion Elliot S. Krames et al 2014 Tiantian Guo et al 2019 Spinal cord anatomy Spinal cord anatomy Source: Prometheus • 3 ascending pathways Anatomical atlas • 2 descending pathways Source: Wikipedia 40-year-old woman So what is this? • gradual and uniform onset of • Lichtheim's disease • diminished pressure, vibration and touch sense • Lou Gehrig's disease • tingling and numbness of legs, arms and trunk that progressively worsens • Lyme disease • pain and temperature sense are normal • Devic’s disease • motor function is preserved, but slight ataxia And the answer is ... Let’s go back ... • gradual and uniform onset of • Lichtheim's disease • diminished pressure, vibration and touch sense • Lou Gehrig's disease • tingling and numbness of legs, arms and trunk that progressively worsens • Lyme disease • pain and temperature sense are normal • Devic’s disease • motor function is preserved, but slight ataxia Spinothalamic tract Spinothalamic tract • 3 -

Definitions of Traumatic Conus Medullaris and Cauda
Spinal Cord (2017) 55, 886–890 & 2017 International Spinal Cord Society All rights reserved 1362-4393/17 www.nature.com/sc ORIGINAL ARTICLE Definitions of traumatic conus medullaris and cauda equina syndrome: a systematic literature review E Brouwers1, H van de Meent2, A Curt3, B Starremans4, A Hosman5 and R Bartels1 Study design: A systematic review. Objectives: Conus medullaris syndrome (CMS) and cauda equina syndrome (CES) are well-known neurological entities. It is assumed that these syndromes are different regarding neurological and functional prognosis. However, literature concerning spinal trauma is ambiguous about the exact definition of the syndromes. Methods: A MEDLINE, EMBASE and Cochrane literature search was performed. We included original articles in which clinical descriptions of CMS and/or CES were mentioned in patients following trauma to the thoracolumbar spine. Results: Out of the 1046 articles, we identified 14 original articles concerning patients with a traumatic CMS and/or CES. Based on this review and anatomical data from cadaveric and radiological studies, CMS and CES could be more precisely defined. Conclusion: CMS may result from injury of vertebrae Th12–L2, and it involves damage to neural structures from spinal cord segment Th12 to nerve root S5. CES may result from an injury of vertebrae L3–L5, and it involves damage to nerve roots L3–S5. This differentiation between CMS and CES is necessary to examine the hypothesis that CES patients tend to have a better functional outcome. Spinal Cord (2017) 55, 886–890; doi:10.1038/sc.2017.54; published online 23 May 2017 INTRODUCTION described clinical symptoms that were caused by compression of the Traumatic injuries of the thoracolumbar spine can result in conus CE and named this as ‘cauda equina compression syndrome’.20 From medullaris syndrome (CMS) or cauda equina syndrome (CES). -

Cauda Equina Syndrome | the BMJ 23/11/2019, 12�15
Cauda equina syndrome | The BMJ 23/11/2019, 1215 Intended for healthcare professionals Clinical Review Cauda equina syndrome BMJ 2009; 338 doi: https://doi-org.sheffield.idm.oclc.org/10.1136/bmj.b936 (Published 31 March 2009) Cite this as: BMJ 2009;338:b936 Chris Lavy, honorary professor and consultant orthopaedic surgeon, Andrew James, specialist registrar, James Wilson-MacDonald, consultant spine surgeon, Jeremy Fairbank, professor of spine surgery 1 Nuffield Department of Orthopaedic Surgery, Nuffield Orthopaedic Centre, Oxford OX3 7LD Correspondence to: C Lavy [email protected] Summary points Cauda equina syndrome is rare, but devastating if symptoms persist Clinical diagnosis is not easy and even in experienced hands is associated with a 43% false positive rate The investigation of choice is magnetic resonance imaging Once urinary retention has occurred the prognosis is worse Good retrospective evidence supports urgent surgery especially in early cases Litigation is common when the patient has residual symptoms An understanding of cauda equina syndrome is important not only to orthopaedic surgeons and neurosurgeons but also to general practitioners, emergency department staff, and other specialists to whom these patients present. Recognition of the syndrome by all groups of clinicians is often delayed as it presents with bladder, bowel, and sexual problems, which are common complaints and have a variety of causes. Patients may not mention such symptoms because of embarrassment or because the onset is slow and insidious. Cauda -

Cauda Equina Or Mower Motor Neurone Injuries
Queensland Spinal Cord Fact Sheet Injuries Service Cauda Equina or Lower Motor Neuron Injuries SPINAL INJURIES UNIT Ph: 3176 2215 This fact sheet provides general information on some of the changes someone may experience Fax: 3176 5061 as a result of having a Lower Motor Neuron Injury. Please note there is additional information provided via hyperlinks throughout this document. These links will redirect to the Queensland OUTPATIENT DEPARTMENT Spinal Cord Injuries Service (QSCIS) website. Ph: 3176 2641 Fax: 3176 5644 Basic Definition of a Lower Motor Neuron (LMN) Injury A lower motor neuron (LMN) injury can result from a Postal and Location cauda equina injury or conus injury. In the lumbar region Princess Alexandra Hospital Ipswich Rd of the spine, there is a spray of spinal nerve roots called Woolloongabba QLD 4102 the cauda equina. Cauda equina in Latin means the AUSTRALIA horse’s tail. A conus injury is a similar injury but is higher up in the TRANSITIONAL cord around L1 or L2 level at the level of the conus of the REHABILITATION PROGRAM cord. This injury may be seen as a mixed presentation of Ph: 3176 9508 an upper motor neuron (UMN) and LMN injury. (See Fax: 3176 9514 picture opposite) Email [email protected] The LMN lesion presents with flaccid or no tone and minimal or nil reflexes (floppy). Other nerve roots in the Postal PO Box 6053 lumbar region can also be damaged. Buranda, QLD, 4102 What happens as a result of the injury? Location A LMN injury is accompanied by a range of symptoms, rd 3 Floor, Buranda Village the severity of which depend on how badly the nerve roots are damaged and which ones are Cnr Cornwall St & Ipswich Rd Buranda, QLD, 4102 damaged. -

The Spinal Cord and Spinal Nerves
14 The Nervous System: The Spinal Cord and Spinal Nerves PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • The Central Nervous System (CNS) consists of: • The spinal cord • Integrates and processes information • Can function with the brain • Can function independently of the brain • The brain • Integrates and processes information • Can function with the spinal cord • Can function independently of the spinal cord © 2012 Pearson Education, Inc. Gross Anatomy of the Spinal Cord • Features of the Spinal Cord • 45 cm in length • Passes through the foramen magnum • Extends from the brain to L1 • Consists of: • Cervical region • Thoracic region • Lumbar region • Sacral region • Coccygeal region © 2012 Pearson Education, Inc. Gross Anatomy of the Spinal Cord • Features of the Spinal Cord • Consists of (continued): • Cervical enlargement • Lumbosacral enlargement • Conus medullaris • Cauda equina • Filum terminale: becomes a component of the coccygeal ligament • Posterior and anterior median sulci © 2012 Pearson Education, Inc. Figure 14.1a Gross Anatomy of the Spinal Cord C1 C2 Cervical spinal C3 nerves C4 C5 C 6 Cervical C 7 enlargement C8 T1 T2 T3 T4 T5 T6 T7 Thoracic T8 spinal Posterior nerves T9 median sulcus T10 Lumbosacral T11 enlargement T12 L Conus 1 medullaris L2 Lumbar L3 Inferior spinal tip of nerves spinal cord L4 Cauda equina L5 S1 Sacral spinal S nerves 2 S3 S4 S5 Coccygeal Filum terminale nerve (Co1) (in coccygeal ligament) Superficial anatomy and orientation of the adult spinal cord. The numbers to the left identify the spinal nerves and indicate where the nerve roots leave the vertebral canal. -

Cauda Equina Syndrome As a Postoperative Complication of Lumbar Spine Surgery
Neurosurg Focus 16 (6):e7, 2004, Click here to return to Table of Contents Cauda equina syndrome as a postoperative complication of lumbar spine surgery RANDY L. JENSEN, M.D., PH.D. Department of Neurosurgery, University of Utah, Salt Lake City, Utah Object. The term “cauda equina syndrome” (CES) has been used to describe the signs and symptoms in patients with compressive neuropathy of multiple lumbar and sacral roots. This syndrome is well known as an indication for surgical intervention in treating lumbar spine disease, but relatively unknown as a postoperative complication follow- ing surgery for disease. In this study the author describes two cases of CES that occurred following uneventful lumbar spine procedures—one microdiscectomy and one decompressive laminectomy. Methods. Preoperative, operative, and postoperative management is discussed and the relevant literature reviewed. One patient suffered perineal numbness and bowel and bladder difficulty following a decompressive laminectomy. Postoperative imaging studies were negative for residual lesion and the treatment goal pursued was partial long-term resolution of symptoms. The second patient had progressive numbness and weakness in the lower extremities. Results of urgent postoperative magnetic resonance imaging studies were inconclusive and repeated exploration was per- formed within hours of the initial procedure. The patient made a full recovery, although the intraoperative findings did not reveal a clear cause of the patient’s symptoms. Conclusions. Postoperative symptoms of partial or complete CES represent a medical emergency, especially if they are progressive. It is necessary to perform urgent postoperative imaging in patients, but the results are not always help- ful. Surgical exploration is warranted if a mass lesion is demonstrated on imaging studies or if symptoms progress and the disease origin is not clear based on available information. -

A Case of Cauda Equina Syndrome Caused by a Simple Sneeze
Osteopathic Family Physician (2011) 3, 27-29 A case of cauda equina syndrome caused by a simple sneeze Doré DeBartolo, DO From the Department of Family Medicine, Advocate Christ Medical Center, Hometown, IL. KEYWORDS: Cauda equina syndrome is a rare but serious condition that is brought on by an acute disc herniation; Cauda equina however, it may also occur in the patient with chronic back pain. The latter case complicates the syndrome; diagnosis for a variety of reasons, especially if the diagnosis is not considered. Reported here is the case Lumbar disc of a patient with longstanding spinal stenosis and lumbar disc herniations who developed an acute case herniation of cauda equina syndrome after a sneeze. Missing the diagnosis can result in significant morbidity including paraplegia and permanent urinary incontinence. This report reviews the definition, causes, diagnosis, and a brief literature review of the timing of treatment of cauda equina syndrome. © 2011 Elsevier Inc. All rights reserved. Introduction incontinence is also a late finding as it is caused by overflow from urinary retention. The first case was reported in 1934 by Low back pain with radicular symptoms is a common com- Dandy,2 although in 1934 the concept was popularized by plaint. The typical treatment is a course of anti-inflamma- Mixter and Barr.3 The majority of patients have a history of tory medication, physical therapy, and epidural spinal in- chronic back pain (about 70%) versus 30% of patients who jections. Failure of these treatment modalities may then present with cauda equina syndrome as a primary manifesta- require the need for surgical decompression.