Thuja Occidentalis) Forests
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Habitat and Microsite Influence Demography of Two
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2013 Population Dynamics And Environmental Factors Influencing Herbs In Intact And Degraded Florida Rosemary Scrub Elizabeth Stephens University of Central Florida Part of the Biology Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Stephens, Elizabeth, "Population Dynamics And Environmental Factors Influencing Herbs In Intact And Degraded Florida Rosemary Scrub" (2013). Electronic Theses and Dissertations, 2004-2019. 2822. https://stars.library.ucf.edu/etd/2822 POPULATION DYNAMICS AND ENVIRONMENTAL FACTORS INFLUENCING HERBS IN INTACT AND DEGRADED FLORIDA ROSEMARY SCRUB by ELIZABETH L. STEPHENS M.S. Purdue University, 2005 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy of Science in the Department of Biology in the College of Sciences at the University of Central Florida Orlando, Florida Spring Term 2013 Major Professor: Pedro F. Quintana-Ascencio © 2013 Elizabeth L. Stephens ii ABSTRACT Species have complex and contextual relationships with their environment; both the relative contributions of life-history stages to population growth and the effect of environmental factors on each stage can be different among co-existing species. Timing and extent of reproduction, survival, and mortality determine population growth, species distributions, and assemblage patterns. I evaluate the role of habitat (intact, degraded) and microsite (shrub, leaf litter, bare sand) on population dynamics of Florida scrub herbs. -

American Forests National Big Tree Program Species Without Champions
American Forests National Big Tree Program Champion trees are the superstars of their species — and there are more than 700 of them in our national register. Each champion is the result of a lucky combination: growing in a spot protected by the landscape or by people who have cared about and for it, good soil, the right amount of water, and resilience to the elements, surviving storms, disease and pests. American Forests National Big Tree Program was founded to honor these trees. Since 1940, we have kept the only national register of champion trees (http://www.americanforests.org/explore- forests/americas-biggest-trees/champion-trees-national-register/) Champion trees are found by people just like you — school teachers, kids fascinated by science, tree lovers of all ages and even arborists for whom a fun day off is measuring the biggest tree they can find. You, too, can become a big tree hunter and compete to find new champions. Species without Champions (March, 2018) Gold rows indict species that have Idaho State Champions but the nominations are too old to be submitted for National Champion status. Scientific Name Species Common Name Abies lasiocarpa FIR Subalpine Acacia macracantha ACACIA Long-spine Acacia roemeriana CATCLAW Roemer Acer grandidentatum MAPLE Canyon or bigtooth maple Acer nigrum MAPLE Black Acer platanoides MAPLE Norway Acer saccharinum MAPLE Silver Aesculus pavia BUCKEYE Red Aesculus sylvatica BUCKEYE Painted Ailanthus altissima AILANTHUS Tree-of-heaven Albizia julibrissin SILKTREE Mimosa Albizia lebbek LEBBEK Lebbek -

Molecular Phylogeny of Chinese Thuidiaceae with Emphasis on Thuidium and Pelekium
Molecular Phylogeny of Chinese Thuidiaceae with emphasis on Thuidium and Pelekium QI-YING, CAI1, 2, BI-CAI, GUAN2, GANG, GE2, YAN-MING, FANG 1 1 College of Biology and the Environment, Nanjing Forestry University, Nanjing 210037, China. 2 College of Life Science, Nanchang University, 330031 Nanchang, China. E-mail: [email protected] Abstract We present molecular phylogenetic investigation of Thuidiaceae, especially on Thudium and Pelekium. Three chloroplast sequences (trnL-F, rps4, and atpB-rbcL) and one nuclear sequence (ITS) were analyzed. Data partitions were analyzed separately and in combination by employing MP (maximum parsimony) and Bayesian methods. The influence of data conflict in combined analyses was further explored by two methods: the incongruence length difference (ILD) test and the partition addition bootstrap alteration approach (PABA). Based on the results, ITS 1& 2 had crucial effect in phylogenetic reconstruction in this study, and more chloroplast sequences should be combinated into the analyses since their stability for reconstructing within genus of pleurocarpous mosses. We supported that Helodiaceae including Actinothuidium, Bryochenea, and Helodium still attributed to Thuidiaceae, and the monophyletic Thuidiaceae s. lat. should also include several genera (or species) from Leskeaceae such as Haplocladium and Leskea. In the Thuidiaceae, Thuidium and Pelekium were resolved as two monophyletic groups separately. The results from molecular phylogeny were supported by the crucial morphological characters in Thuidiaceae s. lat., Thuidium and Pelekium. Key words: Thuidiaceae, Thuidium, Pelekium, molecular phylogeny, cpDNA, ITS, PABA approach Introduction Pleurocarpous mosses consist of around 5000 species that are defined by the presence of lateral perichaetia along the gametophyte stems. Monophyletic pleurocarpous mosses were resolved as three orders: Ptychomniales, Hypnales, and Hookeriales (Shaw et al. -

Ilex Mucronata (Formerly Nemopanthus Mucronata) – Mountain Holly, Catberry Pretty Fruits, but Not Palatable/Edible for Humans; Eaten by Birds
Prepared by Henry Mann, Nature Enthusiast/Naturalist For the Pasadena Ski and Nature Park In late summer and in fall, some herbs, shrubs and trees will produce fleshy fruits, some of which are edible, some inedible and some toxic to humans. Because of their detailed structure they have various botanical names such as pomes, drupes, berries, etc., however, commonly we often refer to all fleshy fruits as just berries. Also many dry fruits are produced, but only a few of these will be featured because of their edibility or toxicity. Photos are from the archives of HM except where otherwise indicated. Edibility “Edibility” is a highly variable term with a range of meanings from delicious, to nourishing and somewhat tasty, to edible but not very palatable. A small number of fruits that are edible and even delicious to most, can be non- palatable or even allergenic to a few individuals. Some fleshy fruits which are not very palatable fresh make superb jams, jellies, syrups, wines, etc. when cooked or fermented. Then there are fruits that have distinct toxic properties from mild to deadly. With any food collected and eaten from the wild it is extremely important to be certain of identity. There can be similar appearing fruits that are poisonous. This presentation does not recommend consuming any of the featured fruits. The viewer takes full personal responsibility for anything he or she eats. Vaccinium angustifolium - Low Sweet Blueberry, Lowbush Blueberry. A Newfoundland favorite and staple. Vaccinium vitis-idaea – Partridgeberry, Mountain Cranberry. A commonly sought and utilized Newfoundland fruit. Fragaria virginiana – Wild Strawberry and F. -

Burton V. Barnes As a Forest Botanist
46 THE MICHIGAN BOTANIST Vol. 54 BURTON V.BARNES AS A FOREST BOTANIST Christopher W. Dick Department of Ecology and Evolutionary Biology University of Michigan Ann Arbor, MI 48104 [email protected] Michael R. Penskar and Anton A. Reznicek University of Michigan Herbarium 3600 Varsity Drive Ann Arbor, MI 48108 [email protected] [email protected] A cornerstone of Burt Barnes’ many contributions to forest botany is the well- known and critically acclaimed course known simply as “Woody Plants.” Cre- ated in 1965 with Burt’s mentor and botanical colleague, Dr. Warren “Herb” Wagner, Jr., and Terry Sharik, then a Graduate Fellow (see Sharik, this issue), the course’s roots trace to a long-standing, entry-level dendrology class. Previously taught primarily to undergraduate forestry students, the dendrology course was formulaic and limited in scope (as well as reportedly quite tedious). Barnes and Wagner used their new course to reframe forest botany and revitalize its peda- gogy with many teaching innovations. Their creativity in organization and in- struction vastly broadened the course’s appeal and the size and diversity of its au- dience. In the mid-1970s the course was designated as a university-wide course and accommodated as many as 200 students per term. Many middle-aged Ann Arbor residents fondly tell of taking the class when they encounter current Woody Plants students in the field. A critical element in the evolution of Woody Plants was fusing the particular specialties that Barnes and Wagner each brought to the course, providing a unique combination of ecological and organismal knowledge delivered via a carefully planned, tag-team teaching format. -

Microhabitat Heterogeneity Enhances Soil Macrofauna and Plant Species Diversity in an Ash – Field Maple Woodland
Title Microhabitat heterogeneity enhances soil macrofauna and plant species diversity in an Ash – Field Maple woodland Authors Burton, VJ; Eggleton, P Description publisher: Elsevier articletitle: Microhabitat heterogeneity enhances soil macrofauna and plant species diversity in an Ash – Field Maple woodland journaltitle: European Journal of Soil Biology articlelink: http://dx.doi.org/10.1016/j.ejsobi.2016.04.012 content_type: article copyright: © 2016 Elsevier Masson SAS. All rights reserved. Date Submitted 2016-07-15 1 Microhabitat heterogeneity enhances soil macrofauna and plant species diversity in an Ash - Field 2 Maple woodland 3 4 Victoria J. Burtonab*, Paul Eggletona 5 aSoil Biodiversity Group, Life Sciences Department, The Natural History Museum, Cromwell Road, 6 London SW7 5BD, UK 7 bImperial College London, South Kensington Campus, London SW7 2AZ, UK 8 *corresponding author email [email protected] 9 10 Abstract 11 The high biodiversity of soil ecosystems is often attributed to their spatial heterogeneity at multiple 12 scales, but studies on the small-scale spatial distribution of soil macrofauna are rare. This case study 13 of an Ash-Field Maple woodland partially converted to conifer plantation investigates differences 14 between species assemblages of soil and litter invertebrates, and plants, using multivariate 15 ordination and indicator species analysis for eleven microhabitats. 16 Microhabitats representing the main body of uniform litter were compared with more localised 17 microhabitats including dead wood and areas of wet soil. Species accumulation curves suggest that 18 for this site it is more efficient to sample from varied microhabitats of limited spatial scale rather 19 than the broad habitat areas when generating a species inventory. -
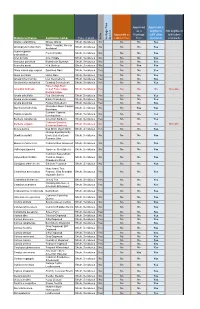
Botanical Name Common Name
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -

Atlantic Woodlands in the Lake District Mosses & Liverworts
LIVERWORTS generally have two rows of leaves, one up each side of the stem We are Plantlife Further information For over 25 years, Plantlife has had a single ideal Bazzania trilobata Greater whipwort Plagiochila spinulosa Prickly featherwort – to save and celebrate wild flowers, plants and Books fungi. They are the life support for all our wildlife Mosses and Liverworts of Britain and Ireland: A Field Guide 3 and their colour and character light up our by Ian Atherton, Sam Bosanquet and Mark Lawley (British landscapes. But without our help, this priceless Bryological Society, 2010). The best field guide. 2 natural heritage is in danger of being lost. The Liverwort Flora of the British Isles by Jean Paton (Harley 3 Books, 1999). An in-depth guide to liverwort identification, From the open spaces of our nature reserves to for more advanced bryologists. the corridors of government, we work nationally The Moss Flora of Britain and Ireland by A J E Smith and internationally to raise their profile, (2nd edition, Cambridge University Press, 2004). 1 celebrate their beauty and to protect their future. An in-depth guide to liverwort identification, for more advanced bryologists. 2 Mosses and Liverworts by Ron Porley and Nick Hodgetts Where wild plants lead (Collins New Naturalist series, Harper Collins, 2005). Habitat Often forming dense, mounded colonies on earth banks and around tree bases; also on boulders, rotten wood and Habitat In loose mats or dense cushions on trees and rock faces, often close to rivers and streams. Wildlife follows A highly readable account of moss and liverwort ecology occasionally on tree trunks. -

<I>Sphagnum</I> Peat Mosses
ORIGINAL ARTICLE doi:10.1111/evo.12547 Evolution of niche preference in Sphagnum peat mosses Matthew G. Johnson,1,2,3 Gustaf Granath,4,5,6 Teemu Tahvanainen, 7 Remy Pouliot,8 Hans K. Stenøien,9 Line Rochefort,8 Hakan˚ Rydin,4 and A. Jonathan Shaw1 1Department of Biology, Duke University, Durham, North Carolina 27708 2Current Address: Chicago Botanic Garden, 1000 Lake Cook Road Glencoe, Illinois 60022 3E-mail: [email protected] 4Department of Plant Ecology and Evolution, Evolutionary Biology Centre, Uppsala University, Norbyvagen¨ 18D, SE-752 36, Uppsala, Sweden 5School of Geography and Earth Sciences, McMaster University, Hamilton, Ontario, Canada 6Department of Aquatic Sciences and Assessment, Swedish University of Agricultural Sciences, SE-750 07, Uppsala, Sweden 7Department of Biology, University of Eastern Finland, P.O. Box 111, 80101, Joensuu, Finland 8Department of Plant Sciences and Northern Research Center (CEN), Laval University Quebec, Canada 9Department of Natural History, Norwegian University of Science and Technology University Museum, Trondheim, Norway Received March 26, 2014 Accepted September 23, 2014 Peat mosses (Sphagnum)areecosystemengineers—speciesinborealpeatlandssimultaneouslycreateandinhabitnarrowhabitat preferences along two microhabitat gradients: an ionic gradient and a hydrological hummock–hollow gradient. In this article, we demonstrate the connections between microhabitat preference and phylogeny in Sphagnum.Usingadatasetof39speciesof Sphagnum,withan18-locusDNAalignmentandanecologicaldatasetencompassingthreelargepublishedstudies,wetested -

Prescribed Fire, Soil, and Plants: Burn Effects and Interactions in the Central Great Basin Benjamin M
Rangeland Ecol Manage 61:169–181 | March 2008 Prescribed Fire, Soil, and Plants: Burn Effects and Interactions in the Central Great Basin Benjamin M. Rau,1 Jeanne C. Chambers,2 Robert R. Blank,3 and Dale W. Johnson4 Authors are 1Graduate Research Assistant, University of Nevada, Reno, Hydrolologic Sciences Program, 1000 Valley Rd, Reno, NV 89512, USA; 2Research Ecologist, USDA Forest Service RMRS, 920 Valley Rd, Reno, NV 89512, USA; 3Soil Scientist, USDA ARS, 920 Valley Rd, Reno, NV 89512, USA; and 4Soil Scientist, University of Nevada, Reno, Department of Natural Resources and Environmental Science, 1000 Valley Rd, Reno, NV 89512, USA. Abstract Pinyon and juniper expansion into sagebrush ecosystems results in decreased cover and biomass of perennial grasses and forbs. We examine the effectiveness of spring prescribed fire on restoration of sagebrush ecosystems by documenting burn effects on soil nutrients, herbaceous aboveground biomass, and tissue nutrient concentrations. This study was conducted in a central Nevada woodland and included control and burn treatment plots sampled before and after a prescribed fire. Six native understory plant species (Crepis acuminata, Eriogonum umbellatum, Eriogonum elatum, Poa secunda secunda, Festuca idahoensis, and Lupinus argenteus) important for native sagebrush obligate foragers were chosen to represent the understory plant community. L. argenteus is also important for system nutrient cycling and nitrogen fixation. Plants were collected from three microsites (under tree canopy, under shrub canopy, and interspace) common in transitional woodlands during peak growth the summer before a spring prescribed burn and each of two summers following the burn. Soils were collected from corresponding locations at two depth intervals (0–8 and 8–52 cm) to determine the relationships between soil and plant nutrients following fire. -

NATURAL RESOURCES a S S O C I a T E O F S C I E N C E I N N a T U R a L R E S O U R C E S – 90 C R E D I T S
NATURAL RESOURCES ASSOCIATE OF SCIENCE IN NATURAL RESOURCES – 90 CREDITS CAREER DESCRIPTION ACCEPTANCE REQUIREMENTS Once the AS degree is completed, there are a number of other UCC courses that meet additional requirements for a The Associate of Science degree in Natural Resources gives Students are required to take college placement tests to Bachelor of Science degree at OSU or that can be used for students a comprehensive educational foundation for determine skill level and readiness for college-level individual specialization options. Consult your advisor to careers related to natural resource science and technology. courses. Coursework from accredited high schools, learn more about these. This program prepares students for jobs in conservation colleges and universities will be accepted in accordance OSU Baccalaureate Core Equivalents: science, wildlife biology, fisheries science, botany, forestry, with college policies and with the approval of the Science BI 211 Principles of Biology I1 5 ecosystem management, watershed management and other Department Chair. BI 212 Principles of Biology II1 5 fields related to natural resource science and conservation. BI 213 Principles of Biology III1 5 The program takes advantage of the diversity of resources PROGRAM INFORMATION ENG 230 Environmental Literature 4 available on nearby public lands, and the expertise of local 1 Students who graduate with an Associate of Science degree G 221 Environmental Geology 4 professionals who manage those lands, to provide a mix of in Natural Resources will be well-trained for entry-level jobs MTH 111 College Algebra 5 classroom, lab and field experiences not found at any other 1 in the natural resource economy. -

Climate Change: the IPCC 1990 and 1992 Assessments 1
CLIMATE CHANGE: The IPCC 1990 and 1992 Assessments CLIMATE CHANGE: The 1990 and 1992 IPCC Assessments IPCC First Assessment Report Overview and Policymaker Summaries and 1992 IPPC Supplement June. 1992 Published with the support of:* Australia Austria Canada France Germany Japan The Netherlands Norway Spain United Kingdom United States of America WMO UNEP © Intergovernmental Panel on Climate Change 1992 Printed in Canada Climate Change: The IPCC 1990 and 1992 Assessments 1. Climate Changes I. Title II. IPCC ISBN: 0-662-19821-2 ® Tills paper contains a minimum of 60% recyded fibres, mduding 10%posn»nsumei fibres ACKNOWLEDGEMENTS Cover Photographs Top Image A composite colour image of GOES satellite using visible and infrared channels. This image was produced by the Data Integration Division, Climate Adaptation Branch, Canadian Climate Centre. Middle Image A full global disc satellite image (Channel Visible 2) for 4 September 1983 reproduced with the permission of EUMETSAT. Bottom Image A full earth disc view of cloud patterns over the Australian region on 19 February 1991 from the Japanese Geostationary Satellite (GMS4). This image is a colour enhanced composite of information from the visible and infrared channels produced by the Australian Centre for Remote Sensing of the Australian Survey and Land Information Group. *Notes Spain - Instituto Nacional de Meteorología üi TABLE OF CONTENTS Preface vü Foreword ix 1992 IPCC Supplement 1 IPCC First Assessment Report: 47 Overview 51 Policymaker Summary of Working Group I (Scientific Assessment