Study on the Prevention of Infection in Terminal Cancer Patients Applying
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modeling and Simulation of Shanghai MAGLEV Train Transrapid with Random Track Irregularities
Modeling and Simulation of Shanghai MAGLEV Train Transrapid with Random Track Irregularities Prof. Shu Guangwei M.Sc. Prof. Dr.-Ing. Reinhold Meisinger Prof. Shen Gang Ph.D. Shanghai Institute of Technology, Shanghai, P.R. China Nuremberg University of Applied Sciences, Nuremberg, Germany Tongji University, Shanghai, P.R. China Abstract The MAGLEV Transrapid is a kind of new type high speed train in the world which is levitated and gui- ded over the track using electro magnetic forces. Because the electro magnets are unstable, they ha- ve to be controlled. Since 2002 the worldwide first commercial use of such a high speed train based on German technology is running successfully in Shanghai Pudong Airport, P.R.China. In this paper modeling of the high speed MAGLEV train Transrapid is discussed, which considered the whole mechanical system of one vehicle with optimized suspension parameters and all controlled electro magnet pairs in vertical and lateral directions. The dynamical simulation code is generated with MATLAB/SIMILINK. For the design of the control system, the optimal Linear Quadratic Control for minimum control energy is used for each single electro magnet. The simulation results are presen- ted with the given vertical and lateral random track irregularities. The research work was carried out together with Prof. Shen Gang, Ph.D. during the time Prof. Dr. Meisinger was visiting professor in Shanghai 2006 and Prof. Shu Guangwei, M.Sc. was visiting profes- sor in Nuremberg 2007. ISSN 1616-0762 Sonderdruck Schriftenreihe der Georg-Simon-Ohm-Fachhochschule Nürnberg Nr. 39, Juli 2007 Schriftenreihe Georg-Simon-Ohm-Fachhochschule Nürnberg Seite 3 1. -

Mandarin Oriental Pudong, Shanghai Celebrates Opening With
MANDARIN ORIENTAL PUDONG, SHANGHAI CELEBRATES OPENING WITH ARTISTIC FLAIR AND TWO TEMPTING OFFERS Mandarin Oriental’s newest luxury landmark on the east bank of the Huangpu River debuts with the most extensive hotel collection of Chinese contemporary art in Shanghai, and two tantalizing opening offers Hong Kong, 25 April, 2013 -- Mandarin Oriental Hotel Group is delighted to announce the opening of its second city hotel in China, Mandarin Oriental Pudong, Shanghai. In celebration, the hotel is offering guests two tantalizing packages both priced from CNY 3,600 and available from 25 April to 30 September 2013. Mandarin Oriental Pudong, Shanghai celebrated its opening with the official launch of the hotel’s impressive art collection, curated by the renowned Art Front Gallery and featuring 4,000 original artworks displayed throughout the public spaces and guestrooms. Joining the celebrations were Associate Professor Li Xiao Feng of Fine Arts College Shanghai University, as well as leading Chinese artists Miao Tong, Pan Wei and Kang Qing. “We are delighted to open our newest hotel in this dynamic city,” said Andrew Hirst, Director of Operations – Asia. “Mandarin Oriental Pudong, Shanghai is set to become a new luxury landmark for guests seeking the finest in service and facilities, as well as a riverfront destination for art connoisseurs.” “Mandarin Oriental Pudong, Shanghai provides a stunning backdrop from which to showcase some of China’s most exciting artworks, and is sure to become a destination for both local and international art lovers,” -
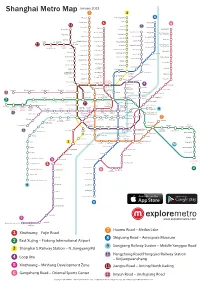
Shanghai Metro Map 7 3
January 2013 Shanghai Metro Map 7 3 Meilan Lake North Jiangyang Rd. 8 Tieli Rd. Luonan Xincun 1 Shiguang Rd. 6 11 Youyi Rd. Panguang Rd. 10 Nenjiang Rd. Fujin Rd. North Jiading Baoyang Rd. Gangcheng Rd. Liuhang Xinjiangwancheng West Youyi Rd. Xiangyin Rd. North Waigaoqiao West Jiading Shuichan Rd. Free Trade Zone Gucun Park East Yingao Rd. Bao’an Highway Huangxing Park Songbin Rd. Baiyin Rd. Hangjin Rd. Shanghai University Sanmen Rd. Anting East Changji Rd. Gongfu Xincun Zhanghuabang Jiading Middle Yanji Rd. Xincheng Jiangwan Stadium South Waigaoqiao 11 Nanchen Rd. Hulan Rd. Songfa Rd. Free Trade Zone Shanghai Shanghai Huangxing Rd. Automobile City Circuit Malu South Changjiang Rd. Wujiaochang Shangda Rd. Tonghe Xincun Zhouhai Rd. Nanxiang West Yingao Rd. Guoquan Rd. Jiangpu Rd. Changzhong Rd. Gongkang Rd. Taopu Xincun Jiangwan Town Wuzhou Avenue Penpu Xincun Tongji University Anshan Xincun Dachang Town Wuwei Rd. Dabaishu Dongjing Rd. Wenshui Rd. Siping Rd. Qilianshan Rd. Xingzhi Rd. Chifeng Rd. Shanghai Quyang Rd. Jufeng Rd. Liziyuan Dahuasan Rd. Circus World North Xizang Rd. Shanghai West Yanchang Rd. Youdian Xincun Railway Station Hongkou Xincun Rd. Football Wulian Rd. North Zhongxing Rd. Stadium Zhenru Zhongshan Rd. Langao Rd. Dongbaoxing Rd. Boxing Rd. Shanghai Linping Rd. Fengqiao Rd. Zhenping Rd. Zhongtan Rd. Railway Stn. Caoyang Rd. Hailun Rd. 4 Jinqiao Rd. Baoshan Rd. Changshou Rd. North Dalian Rd. Sichuan Rd. Hanzhong Rd. Yunshan Rd. Jinyun Rd. West Jinshajiang Rd. Fengzhuang Zhenbei Rd. Jinshajiang Rd. Longde Rd. Qufu Rd. Yangshupu Rd. Tiantong Rd. Deping Rd. 13 Changping Rd. Xinzha Rd. Pudong Beixinjing Jiangsu Rd. West Nanjing Rd. -

Transportation the Conference Will Be Held in North Zhongshan Road
Transportation The conference will be held in North Zhongshan Road campus of East China Normal University (ECNU). The address is: No. 3663, North Zhongshan Road, Putuo, Shanghai. The hotel where all participants will stay is Yifu Building (逸夫楼) located on the campus. Arrival by flights: There are two airports in Shanghai: the Pudong Airport in east Shanghai and the Hongqiao Airport in west Shanghai. Both are convenient to get to the North Zhongshan Road campus of ECNU. At the airport, you can take taxi or metro to North Zhongshan Road Campus of ECNU. Taxi is recommended in terms of its convenience and time saving. Our students will be there for helping you to find the taxi. By taxi From Pudong Airport: about one hour and 200 CNY when traffic is not busy. From Hongqiao Airport: about 30 minutes and 60 CNY when traffic is not busy. Please print the following tips if you like “请带我去华东师范大学中山北路校区正门” in advance and show it to the taxi driver. The Chinese words in tips mean "Please take me to the main gate of North Zhongshan Road Campus of ECNU". By metro From Pudong Airport: 1. Metro Line 2 (6:00 - 22:00)->Zhongshan Park (中山公园) station (Note: exchange at Guanglan Road station(广兰路)), then take taxi for about 10 minutes and 14 CNY cost to North Zhongshan Road Campus of ECNU, or take the bus 67 for 2 stops and get off at ECNU station(华东师大站)instead. 2. Metro Line 2->Jiangsu Road (江苏路) station-> Metro Line 11->Longde Road (隆德路) station->Metro Line 13-> Jinshajiang Road (金沙江路) Station->10 minutes walk. -

Development of High-Speed Rail in the People's Republic of China
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Haixiao, Pan; Ya, Gao Working Paper Development of high-speed rail in the People's Republic of China ADBI Working Paper Series, No. 959 Provided in Cooperation with: Asian Development Bank Institute (ADBI), Tokyo Suggested Citation: Haixiao, Pan; Ya, Gao (2019) : Development of high-speed rail in the People's Republic of China, ADBI Working Paper Series, No. 959, Asian Development Bank Institute (ADBI), Tokyo This Version is available at: http://hdl.handle.net/10419/222726 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. may exercise further usage rights as specified in the indicated licence. https://creativecommons.org/licenses/by-nc-nd/3.0/igo/ www.econstor.eu ADBI Working Paper Series DEVELOPMENT OF HIGH-SPEED RAIL IN THE PEOPLE’S REPUBLIC OF CHINA Pan Haixiao and Gao Ya No. -

UNIVERSITY of CAMBRIDGE INTERNATIONAL EXAMINATIONS Cambridge International Level 3 Pre-U Certificate Principal Subject
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS Cambridge International Level 3 Pre-U Certificate Principal Subject PHYSICS 9792/02 Paper 2 Part A Written Paper May/June 2012 PRE-RELEASED MATERIAL The question in Section B of Paper 2 will relate to the subject matter in these extracts. You should read through this booklet before the examination. The extracts on the following pages are taken from a variety of sources. University of Cambridge International Examinations does not necessarily endorse the reasoning expressed by the original authors, some of whom may use unconventional Physics terminology and non-SI units. You are also encouraged to read around the topic, and to consider the issues raised, so that you can draw on all your knowledge of Physics when answering the questions. You will be provided with a copy of this booklet in the examination. This document consists of 8 printed pages. DC (LEO/JG) 51113/1 © UCLES 2012 [Turn over 2 Extract 1: How Maglev Trains Work If you’ve been to an airport lately, you’ve probably noticed that air travel is becoming more and more congested. Despite frequent delays, aeroplanes still provide the fastest way to travel hundreds or thousands of miles. Passenger air travel revolutionised the transport industry in the last century, letting people traverse great distances in a matter of hours instead of days or weeks. Fig. E1.1 The first commercial maglev line made its debut in December of 2003. The only alternatives to aeroplanes – feet, cars, buses, boats and conventional trains – are just too slow for today’s fast-paced society. -

Shanghai, China
The case of Shanghai, China by ZHU Linchu and QIAN Zhi Contact: ZHU Linchu and QIAN Zhi Source: CIA factbook The Development Research Centre of Shanghai Municipal Government, No. 200 People's Avenue, Shanghai, 200003, P. R. China Tel.+86 21 63582710 Fax. +86 21 63216751 [email protected] [email protected] I. INTRODUCTION: THE CITY A. CHARACTERISTICS AND TRENDS IN THE URBAN DEVELOPMENT OF SHANGHAI The pace of urbanisation in China since 1978, Shanghai, one of the largest cities in China, sits together with the implementation of the Economic midway down China's coastline, where the country's Reform and Opening Up Policy and rapid economic longest river, the Yangtze, or Chang Jiang, pours into growth, has been fairly fast. Cities - big, medium-sized the sea. The city, at the mouth of the Yangtze River and small - have all undergone a period of construction delta, has the East China Sea to its east, the Hangzhou and redevelopment. Bay to the south, while behind it is the vast span of China's interior landmass. Shanghai's geographical location facilitates all forms of transport, with first-rate Figure 1: Urbanisation in China sea and river ports combined with the huge water trans- portation network, well-developed railways and roads, and two large international airports, which no other Chinese city has. The total area of Shanghai at the end of 2001 was 6,340.5 km2, covering 18 districts, one county, 144 zhen, 3 xiang, 99 sub-districts, 3,407 residents commit- tees, and 2,699 village committees. Shanghai occupies 0.06 per cent of the national area and houses 1.31 per cent of the national population, producing 5.16 per cent of national income. -

By Airport Bus (One Transfer): There Are Several Airport Bus Lines Located Just Outside the Arrival Area of Pudong International Airport
There are two airports in Shanghai: the HongQiao International airport and the Pudong International Airport. From HongQiao International Airport to the ZhongShan North Road campus of ECNU: The distance is about 7miles. You can take a taxi at the HongQiao International Airport (to ECNU). It will cost around 70 RMB(CNY), and it typically takes about 50 minutes’ for the taxi to drive from Shanghai Hongqiao International Airport to East China Normal University. From Pudong International Airport to the ZhongShan North Road campus of ECNU: The distance from Pudong International Airport to ECNU is about 32 miles. You can take a taxi, take the airport bus, take the subway or take the MagLev. By taxi (no transfer): It is about 1.5 hour's taxi drive from Pudong International Airport to ECNU. It will cost around 220 RMB(CNY) in the daytime and around 300 RMB(CNY) at night. By airport bus (one transfer): There are several airport bus lines located just outside the arrival area of Pudong International Airport. Just take Airport Bus Line No. 2 to Jing'an Temple (静安寺), which will cost around 24 RMB(CNY). After arriving at Jing'an Temple, take a taxi to ECNU, which will cost around 30 RMB(CNY). By subway (two transfers): Take the subway line 2 to Zhongshan Park station, then take a taxi or the bus (No. 67) to ECNU. The subway takes about 60 minutes from Pudong International airport to Zhongshan Park stop, with the fare 7 RMB(CNY). You will transfer from 4-carriage train to 8-carriage train at Guanglan Road Station. -

Notice to Passengers of Shanghai Maglev Demonstration Line
Notice to Passengers on Shanghai Maglev Demonstration Line Ⅰ. Operation time: Longyang Road Station : First train: 6:45 Last train: 21:40. Pudong International Airport Station: First train: 7:02 Last train: 21:42. Ⅱ. Ticketing time: Passengers can buy tickets at the Ticket Center just before getting on the maglev train. Ⅲ.Purchase place: Longyang Rd. Ticket Center is located at the 2nd floor, No. 2100 Longyang Road Station, Shanghai. Pudong Airport Ticket Center is located at the 2nd floor of the Pudong International Airport Station. Ⅳ.Fare: Type Economic Class VIP Single-Trip ( valid on the day of 50 RMB 100 RMB issue) Round-Trip 80 RMB 160 RMB ( valid within 7 days) (1)Passengers who bought the single-trip tickets can get on board at Longyang Road Station or Pudong International Airport Station and get off at the other Station. (2)Passengers who bought the round trip tickets can get on board at either the Long yang Road Station or Pudong International Airport Station, get off at the other station and re-enter through the gate. Round trip tickets are valid within 7 days since the purchase day. (3)Each Passenger with the airplane ticket of the same day can obtain a 20% discount for only one single-trip ticket. (4)Disabled people with the disabled cards can obtain a 20% discount for one single-trip ticket or one round-trip ticket. (5)Each adult can bring one child at or under 130cm free of charge. Children above 130cm shall buy the full-price ticket. Preschoolers are not allowed to take the train alone. -

Five New Towns in Shanghai. Present Situation and Future Perspectives
1 3 Five new towns in Shanghai. 2 Present situation and future perspectives. 4 5 State-of-the Art 2013/2014 Ulf Ranhagen April 2014 Department of Urban Planning at the Swedish Royal Institute of Technology (KTH) © Ulf Ranhagen 2014 Graphic design Olav Heinmets Photos Ulf Ranhagen Printed by US-AB, Universitetsservice TRITA SoM 2014-04 ISSN 1653-6126 ISNR KTH/SoM/14-04/SE ISBN 978-91-7595-068-6 Contents Preface 4 Abstract 5 Introduction 6 The five selected new towns for the study trip 8 Luodian Town – the Swedish New Town 9 Gaoqiao – Holland Village 24 Anting New Town – the German New Town 27 Thames Town – the British New Town 30 Pujiang New Town – the Italian New Town 33 Summary of the analysis of the five new towns 35 Final reflections 38 References 39 Preface I am a Swedish Professor working in the Department of I warmly thank FFNS research foundation for its support of Urban Planning at the Swedish Royal Institute of Technolo- the study. I thank Leon Yu, General Manager of Shanghai gy (KTH) and as Chief Architect at Sweco. I have received Golden Luodian Development Ltd., and Professor Liu Xiao a grant from FFNS research foundation to perform an Ping and Harry den Hartog, author of Shanghai New Towns, overall diagnosis and analysis of the State-of-the-Art of the for giving me valuable information on Luodian New Town. urban environment of new towns in Shanghai, especially The information centers of Anting New Town and Holland the towns developed within the framework of the One City Village also gave me valuable, useful information material for Nine Towns, which was launched in 2001. -

Development of High-Speed Rail in the People's Republic of China
ADBI Working Paper Series DEVELOPMENT OF HIGH-SPEED RAIL IN THE PEOPLE’S REPUBLIC OF CHINA Pan Haixiao and Gao Ya No. 959 May 2019 Asian Development Bank Institute Pan Haixiao is a professor at the Department of Urban Planning of Tongji University. Gao Ya is a PhD candidate at the Department of Urban Planning of Tongji University. The views expressed in this paper are the views of the author and do not necessarily reflect the views or policies of ADBI, ADB, its Board of Directors, or the governments they represent. ADBI does not guarantee the accuracy of the data included in this paper and accepts no responsibility for any consequences of their use. Terminology used may not necessarily be consistent with ADB official terms. Working papers are subject to formal revision and correction before they are finalized and considered published. The Working Paper series is a continuation of the formerly named Discussion Paper series; the numbering of the papers continued without interruption or change. ADBI’s working papers reflect initial ideas on a topic and are posted online for discussion. Some working papers may develop into other forms of publication. Suggested citation: Haixiao, P. and G. Ya. 2019. Development of High-Speed Rail in the People’s Republic of China. ADBI Working Paper 959. Tokyo: Asian Development Bank Institute. Available: https://www.adb.org/publications/development-high-speed-rail-prc Please contact the authors for information about this paper. Email: [email protected] Asian Development Bank Institute Kasumigaseki Building, 8th Floor 3-2-5 Kasumigaseki, Chiyoda-ku Tokyo 100-6008, Japan Tel: +81-3-3593-5500 Fax: +81-3-3593-5571 URL: www.adbi.org E-mail: [email protected] © 2019 Asian Development Bank Institute ADBI Working Paper 959 Haixiao and Ya Abstract High-speed rail (HSR) construction is continuing at a rapid pace in the People’s Republic of China (PRC) to improve rail’s competitiveness in the passenger market and facilitate inter-city accessibility. -

China Shanghai Pudong International Airport Construction Project 1
China Shanghai Pudong International Airport Construction Project Report: December, 2001 Field Survey: August, 2001 1. Project Profile and Japan's ODA Loan Site Map: Shanghai Site Photo: Shanghai Pudong International Airport 1.1 Background It was expected that the annual volume of aerial transportation in China would rapidly increase to reach 100 million passengers and 2 million tons of Cargo by 2000 (the number of passengers was 51 million and the volume of cargo was 1.01 million tons in 1995). To prepare for this increased demand, the Chinese government planned to develop 41 major airports, including this project, and to expand the radar control system through the Ninth Five-Year Plan. Yangzi Shanghai City, in which traffic volume in 1995 reached 11.08 million in passengers (the third highest in China) and 370,000 tons in cargo (the second highest), was regarded as a center city for Hongqiao Airport Pudong JIANGSU New aerial transportation in China. It was expected Area that further economic development in the Pudong New Area, the location of this project, Huangpu Shanghai City would increase the annual volume of aerial transportation in 2005 to 33 million in Pudong District passengers and 1.2 million tons in cargo. ZHEJIANG Although the current Hongqiao Airport had just seen completion of construction for expansion in 1996, it already faced more demands than its capacity could accommodate. In addition, further expansion was considered difficult since it is located in an area of dense population.1 Thus, in order to respond to the increasing aerial demand in the Shanghai District, it became necessary by this project to construct a new airport, mainly for international services, in the Pudong District.