THE EFFECT of URBANISATION on the SUPERB FAIRY-WREN (Malurus Cyaneus)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Systematic Relationships and Biogeography of the Tracheophone Suboscines (Aves: Passeriformes)
MOLECULAR PHYLOGENETICS AND EVOLUTION Molecular Phylogenetics and Evolution 23 (2002) 499–512 www.academicpress.com Systematic relationships and biogeography of the tracheophone suboscines (Aves: Passeriformes) Martin Irestedt,a,b,* Jon Fjeldsaa,c Ulf S. Johansson,a,b and Per G.P. Ericsona a Department of Vertebrate Zoology and Molecular Systematics Laboratory, Swedish Museum of Natural History, P.O. Box 50007, SE-104 05 Stockholm, Sweden b Department of Zoology, University of Stockholm, SE-106 91 Stockholm, Sweden c Zoological Museum, University of Copenhagen, Copenhagen, Denmark Received 29 August 2001; received in revised form 17 January 2002 Abstract Based on their highly specialized ‘‘tracheophone’’ syrinx, the avian families Furnariidae (ovenbirds), Dendrocolaptidae (woodcreepers), Formicariidae (ground antbirds), Thamnophilidae (typical antbirds), Rhinocryptidae (tapaculos), and Conop- ophagidae (gnateaters) have long been recognized to constitute a monophyletic group of suboscine passerines. However, the monophyly of these families have been contested and their interrelationships are poorly understood, and this constrains the pos- sibilities for interpreting adaptive tendencies in this very diverse group. In this study we present a higher-level phylogeny and classification for the tracheophone birds based on phylogenetic analyses of sequence data obtained from 32 ingroup taxa. Both mitochondrial (cytochrome b) and nuclear genes (c-myc, RAG-1, and myoglobin) have been sequenced, and more than 3000 bp were subjected to parsimony and maximum-likelihood analyses. The phylogenetic signals in the mitochondrial and nuclear genes were compared and found to be very similar. The results from the analysis of the combined dataset (all genes, but with transitions at third codon positions in the cytochrome b excluded) partly corroborate previous phylogenetic hypotheses, but several novel arrangements were also suggested. -

Disaggregation of Bird Families Listed on Cms Appendix Ii
Convention on the Conservation of Migratory Species of Wild Animals 2nd Meeting of the Sessional Committee of the CMS Scientific Council (ScC-SC2) Bonn, Germany, 10 – 14 July 2017 UNEP/CMS/ScC-SC2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II (Prepared by the Appointed Councillors for Birds) Summary: The first meeting of the Sessional Committee of the Scientific Council identified the adoption of a new standard reference for avian taxonomy as an opportunity to disaggregate the higher-level taxa listed on Appendix II and to identify those that are considered to be migratory species and that have an unfavourable conservation status. The current paper presents an initial analysis of the higher-level disaggregation using the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World Volumes 1 and 2 taxonomy, and identifies the challenges in completing the analysis to identify all of the migratory species and the corresponding Range States. The document has been prepared by the COP Appointed Scientific Councilors for Birds. This is a supplementary paper to COP document UNEP/CMS/COP12/Doc.25.3 on Taxonomy and Nomenclature UNEP/CMS/ScC-Sc2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II 1. Through Resolution 11.19, the Conference of Parties adopted as the standard reference for bird taxonomy and nomenclature for Non-Passerine species the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World, Volume 1: Non-Passerines, by Josep del Hoyo and Nigel J. Collar (2014); 2. -
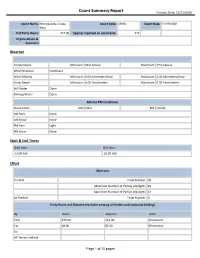
Count Summary Report Printout Date: 11/11/2016
Count Summary Report Printout Date: 11/11/2016 Count Name: Monteverde, Costa Count Code: CRMO Count Date: 12/19/2001 Rica # of Party Hours: 257.00 Species reported on count date: 376 Organizations & Sponsors: Weather Temperature Minimum: 59.0 Celsius Maximum: 77.0 Celsius Wind Direction Northeast Wind Velocity Minimum: 0.00 Kilometers/hour Maximum: 6.00 Kilometers/hour Snow Depth Minimum: 0.00 Centimeters Maximum: 0.00 Centimeters Still Water Open Moving Water Open AM and PM Conditions Cloud Cover AM: Clear PM: Cloudy AM Rain None AM Snow None PM Rain Light PM Snow None Start & End Times Start time End time 12:00 AM 10:00 AM Effort Observers In Field Total Number: 70 Minimum Number of Parties (daylight): 19 Maximum Number of Parties (daylight): 24 At Feeders Total Number: 0 Party Hours and Distance (excludes viewing at feeders and nocturnal birding) By Hours Distance Units Foot 239.00 114.00 Kilometers Car 18.00 85.00 Kilometers Air All-Terrain Vehicle Page 1 of 12 pages Count Summary Report Printout Date: 11/11/2016 Bicycle Dog Sled Golfcart Horseback Motorized Boat Non-Motorized Boat Skis/Xc-Skis Snowmachine Snowshoe Wheelchair Other Time and Distance Hours Distance Units At Feeders 0.00 Nocturnal Birding 17.00 26.00 Miles Total Party 257.00 199.00 Kilometers Checklist Species Number Number/Party Hrs. Flags Editorial Codes Highland Tinamou 3 0.0117 Great Tinamou 2 0.0078 Gray-headed Chachalaca 33 0.1284 Crested Guan 20 0.0778 Black Guan 18 0.0700 Black-breasted Wood-Quail 56 0.2179 Least Grebe 5 0.0195 Anhinga 1 0.0039 Fasciated Tiger-Heron -

Ecology, Morphology, and Behavior in the New World Wood Warblers
Ecology, Morphology, and Behavior in the New World Wood Warblers A dissertation presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Doctor of Philosophy Brandan L. Gray August 2019 © 2019 Brandan L. Gray. All Rights Reserved. 2 This dissertation titled Ecology, Morphology, and Behavior in the New World Wood Warblers by BRANDAN L. GRAY has been approved for the Department of Biological Sciences and the College of Arts and Sciences by Donald B. Miles Professor of Biological Sciences Florenz Plassmann Dean, College of Arts and Sciences 3 ABSTRACT GRAY, BRANDAN L., Ph.D., August 2019, Biological Sciences Ecology, Morphology, and Behavior in the New World Wood Warblers Director of Dissertation: Donald B. Miles In a rapidly changing world, species are faced with habitat alteration, changing climate and weather patterns, changing community interactions, novel resources, novel dangers, and a host of other natural and anthropogenic challenges. Conservationists endeavor to understand how changing ecology will impact local populations and local communities so efforts and funds can be allocated to those taxa/ecosystems exhibiting the greatest need. Ecological morphological and functional morphological research form the foundation of our understanding of selection-driven morphological evolution. Studies which identify and describe ecomorphological or functional morphological relationships will improve our fundamental understanding of how taxa respond to ecological selective pressures and will improve our ability to identify and conserve those aspects of nature unable to cope with rapid change. The New World wood warblers (family Parulidae) exhibit extensive taxonomic, behavioral, ecological, and morphological variation. -

Passerines: Perching Birds
3.9 Orders 9: Passerines – perching birds - Atlas of Birds uncorrected proofs 3.9 Atlas of Birds - Uncorrected proofs Copyrighted Material Passerines: Perching Birds he Passeriformes is by far the largest order of birds, comprising close to 6,000 P Size of order Cardinal virtues Insect-eating voyager Multi-purpose passerine Tspecies. Known loosely as “perching birds”, its members differ from other Number of species in order The Northern or Common Cardinal (Cardinalis cardinalis) The Common Redstart (Phoenicurus phoenicurus) was The Common Magpie (Pica pica) belongs to the crow family orders in various fine anatomical details, and are themselves divided into suborders. Percentage of total bird species belongs to the cardinal family (Cardinalidae) of passerines. once thought to be a member of the thrush family (Corvidae), which includes many of the larger passerines. In simple terms, however, and with a few exceptions, passerines can be described Like the various tanagers, grosbeaks and other members (Turdidae), but is now known to belong to the Old World Like many crows, it is a generalist, with a robust bill adapted of this diverse group, it has a thick, strong bill adapted to flycatchers (Muscicapidae). Its narrow bill is adapted to to feeding on anything from small animals to eggs, carrion, as small birds that sing. feeding on seeds and fruit. Males, from whose vivid red eating insects, and like many insect-eaters that breed in insects, and grain. Crows are among the most intelligent of The word passerine derives from the Latin passer, for sparrow, and indeed a sparrow plumage the family is named, are much more colourful northern Europe and Asia, this species migrates to Sub- birds, and this species is the only non-mammal ever to have is a typical passerine. -

MS1001 Greeney
The nest of the Pearled Treerunner (Margarornis squamiger ) Artículo El nido del Subepalo Perlado (Margarornis squamiger) Harold F. Greeney1 & Rudy A. Gelis2 1Yanayacu Biological Station & Center for Creative Studies, Cosanga, Napo Province, Ecuador, c/o 721 Foch y Amazonas, Quito, Ecuador. [email protected] 2Pluma Verde Tours, Pasaje Manuel Garcia y 18 de Septiembre N20-28 Quito, Ecuador. Abstract Ornitología Colombiana Ornitología The Pearled Treerunner (Margarornis squamiger) is a small ovenbird (Furnariidae) inhabiting the upper strata of Neotropical montane forests. Little is known of its breeding habits despite its wide distribution and abundance within appropriate habitat. The genus Margarornis is considered closely related to Premnoplex barbtails, but details of nest architecture supporting this relationship are unavailable. Here we provide the first detailed description of nest architecture for the Pearled Treerunner from a nest encountered in northwest Ecuador. The nest was a tightly woven ball of moss and rootlets, similar in shape to that of the Spotted Barbtail (Premnoplex brunnescens) and presumably built in a similar manner. Nest architecture and nes- tling behavior support a close relationship between Margarornis and Premnoplex. Key words: barbtail, Ecuador, Furnariidae, Margarornis squamiger, nest architecture. Resumen El Subepalo Perlado (Margarornis squamiger) es un furnárido pequeño que habita el dosel de los bosques de montaña neo- tropicales. La reproducción de esta especie es poco conocida, a pesar de su amplia distribución y de ser común en el hábi- tat adecuado. El género Margarornis se considera cercanamente emparentado con los subepalos del género Premnoplex, pero no se conocen detalles de la arquitectura del nido que sustenten esta relación. -

Wood Warblers Wildlife Note
hooded warbler 47. Wood Warblers Like jewels strewn through the woods, Pennsylvania’s native warblers appear in early spring, the males arrayed in gleaming colors. Twenty-seven warbler species breed commonly in Pennsylvania, another four are rare breeders, and seven migrate through Penn’s Woods headed for breeding grounds farther north. In central Pennsylvania, the first species begin arriving in late March and early April. Louisiana waterthrush (Parkesia motacilla) and black and white warbler (Mniotilta varia) are among the earliest. The great mass of warblers passes through around mid-May, and then the migration trickles off until it ends in late May by which time the trees have leafed out, making it tough to spot canopy-dwelling species. In southern Pennsylvania, look for the migration to begin and end a few days to a week earlier; in northern Pennsylvania, it is somewhat later. As summer progresses and males stop singing on territory, warblers appear less often, making the onset of fall migration difficult to detect. Some species begin moving south as early as mid and late July. In August the majority specific habitat types and show a preference for specific of warblers start moving south again, with migration characteristics within a breeding habitat. They forage from peaking in September and ending in October, although ground level to the treetops and eat mainly small insects stragglers may still come through into November. But by and insect larvae plus a few fruits; some warblers take now most species have molted into cryptic shades of olive flower nectar. When several species inhabit the same area, and brown: the “confusing fall warblers” of field guides. -

The Influence of Environmental Heterogeneity on Winter Ranging in the Red-Backed Fairy-Wren, Malurus Melanocephalus
28 Tulane Undergraduate Research Journal | 2015 The Influence of Environmental Heterogeneity on Winter Ranging in the Red-Backed Fairy-Wren, Malurus melanocephalus Erik N. Iverson, Tulane University Payton M. Phillips, College of William & Mary Abstract The decline in food and cover that accompanies the tropical dry season can cause animals to shift and expand their home ranges. However, range expansion is problematic for territorial species. In birds, this challenge can be overcome via seasonal fission-fusion, a pattern in which birds combine individual breeding season territories in the nonbreeding season and travel as a flock over their combined range. Such behavior is present in a variety of birds and may have environmental, trophic, and social causes. Here we investigate a potential environmental cause in one such bird, the red-backed fairy-wren (RBFW) Malurus melanocephalus. We used spatial analysis of home ranges, movement logs, and fire history to see whether access to unburned habitat was associated with seasonal fission-fusion. RBFWs showed a strong preference for unburned habitat. However, two other predictions—that there would be a positive relationship between home range size and sociality and that flock size would be larger in unburned areas—were not supported. Our results suggest that fusion into large flocks did not occur at our site or had not begun in earnest during the study period. Fusion probably takes off in the late dry season and could potentially be driven by access to unburned habitat. This conclusion is suggested by RBFW's preference for unburned habitat, though other environmental and trophic factors are likely to drive group fusion as well. -

Seiurus Aurocapilla) on VACA KEY, FLORIDA
Florida Field Naturalist 41(4):123-125, 2013. ECTOPARASITES COLLECTED FROM THE OVENBIRD (Seiurus aurocapilla) ON VACA KEY, FLORIDA LAWRENCE J. HRIBAR Florida Keys Mosquito Control District, 503 107th Street, Marathon, Florida 33050 The quill mite, Syringophiloidus seiurus (Clark) (Prostigmata: Syringophilidae) and the louse flyOrnithoctona fusciventris (Wiedemann) (Diptera: Hippoboscidae) are among the very few records of ectoparasites from the Ovenbird, Seiurus aurocapilla from Florida (Forrester and Spaulding 2003). On the 17th of November 2011, an Ovenbird was found dead outside a building on Vaca Key in Marathon, Florida (24.729984, -81.039438), apparently having collided with a plate glass window. The bird was handled and feather mites recovered and prepared for study in the same manner as were the specimens examined by Hribar and Miller (2011). Only twenty-five feather mites were recovered. Slide mounts were examined via phase contrast microscopy and then sent to a specialist for identification. Three mite species were recovered, two Proctophyllodidae (Proctophyllodes sp., Amerodectes sp.) and one Trouessartiidae. Unfortunately no specimens were readily identifiable to species. One female mite was identified as Proctophyllodes sp. Females of this genus are very difficult to identify to species, however,Proctophyllodes breviquadratus Atyeo and Braasch is known from Ovenbirds (Atyeo and Braasch 1966). One male and three female Amerodectes were not identifiable to species and may represent an undescribed species. Amerodectes mites are found on a variety of birds in the New World, viz., Apodiformes: Apodidae; Passeriformes: Cardinalidae, Emberizidae, Furnariidae, Hirundinidae, Icteridae, Parulidae, Thraupidae,and Turdidae (Valim and Hernandes 2010). The two male and two female Trouessartia mites appear to be conspecific with mites found on Ovenbirds in Alberta, Canada, and also represent an undescribed species (H. -

Occurrence of Red-Backed Fairy-Wren and Southern Emu-Wren at Warakeila
Rare wrens at Warakeila The Whistler 1 (2007): 46-48 Occurrence of Red-backed Fairy-wren and Southern Emu-wren at Warakeila Mike Newman 7 Glenurie Close, Woodville, NSW 2321 INTRODUCTION isolated copse of trees (site 3) and flats adjacent to the Allyn River (site 4). The Red-backed Fairy-wren (Malurus melanocephalus) is an uncommon resident species in the north of the Hunter region (Stuart 2005). OBSERVATIONS Dungog (32o 25´S 151o 45´E) is considered to be the southern limit of its distribution in coastal NSW Red-backed Fairy-wren although at the time of European colonisation of Australia records provided by early paintings The Red-backed Fairy-wren was first recorded at suggest that the species occurred as far south as Warakeila in April 2001 when 3 birds, including a Sydney (McAllan 2002). The Southern Emu-wren male in eclipse plumage were seen in a dry creek (Stipiturus malachurus), is an elusive species which bed at the top of the property. The species has been is locally common in the Hunter region (Stuart recorded during five subsequent surveys including 2005). This note places on record observations of every season with the number recorded varying the Red-backed Fairy-wren and Southern Emu-wren from one to five. All observations have been from at Warakeila (32o 15´S 151o 31´E) and compares the vicinity of the original record or from another them with records of the more common Superb dry creek bed running down the property within (Malurus cyaneus) and Variegated (Malurus 1km of the original location (Figure 1). -

Different Modes of Evolution in Males and Females Generate Dichromatism in Fairy-Wrens (Maluridae)
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications in the Biological Sciences Papers in the Biological Sciences 2013 Different modes of evolution in males and females generate dichromatism in fairy-wrens (Maluridae) Allison E. Johnson J. Jordan Price Stephen Pruett-Jones Follow this and additional works at: https://digitalcommons.unl.edu/bioscifacpub Part of the Biology Commons, and the Ornithology Commons This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications in the Biological Sciences by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Different modes of evolution in males and females generate dichromatism in fairy-wrens (Maluridae) Allison E. Johnson1, J. Jordan Price2 & Stephen Pruett-Jones1 1Department of Ecology and Evolution, The University of Chicago, Chicago, Illinois 60637-1503 2Department of Biology, St. Mary’s College of Maryland, St. Mary’s City, Maryland 20686-3001 Keywords Abstract Fairy-wrens, Maluridae, plumage evolution, selection, sexual dimorphism. Sexual dichromatism in birds is often attributed to selection for elaboration in males. However, evolutionary changes in either sex can result in plumage differ- Correspondence ences between them, and such changes can result in either gains or losses of Allison Johnson, Department of Ecology and dimorphism. We reconstructed the evolution of plumage colors in both males Evolution, University of Chicago, 1101 East and females of species in Maluridae, a family comprising the fairy-wrens (Malurus, 57th St., Chicago, IL 60637. Tel: 773-702- Clytomias, Sipodotus), emu-wrens (Stipiturus), and grasswrens (Amytornis). -

The Behavioural Ecology of the Thick-Billed Grasswren
The behavioural ecology of the thick-billed grasswren Marina (Maria Carolina Johanna) Louter (MSc Biology) A thesis submitted in fulfilment of the requirements for the Degree of Doctor of Philosophy School of Biological Sciences Faculty of Science and Engineering Flinders University of South Australia Cover image: Typical thick-billed grasswren habitat with chenopod shrubs at Witchelina Nature Reserve in South Australia, and (inset) a thick-billed grasswren (Amytornis modestus raglessi) in the hand. Photos by Marina Louter. ii Table of Contents List of Tables ................................................................................................................... vii List of Figures ................................................................................................................... ix List of Supplementary Material ..................................................................................... xi Thesis Summary .............................................................................................................. xii Declaration...................................................................................................................... xiv Acknowledgements ......................................................................................................... xv Statement of Authorship/Contribution and Acknowledgment ............................... xviii Chapter 1 General introduction ................................................................ 1 Behavioural conservation framework ...................................................................