Study on Groundwater Analysis for Drinking Purpose in Mangalagiri
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

E-Auction Sale Notice
SALE NOTICE FOR SALE OF IMMOVABLE PROPERTIES [Under Rule 8(6) of Security Interest {Enforcement} Rules] E-Auction Sale Notice for Sale of Immovable Assets under the Securitization and Reconstruction of Financial Assets and Enforcement of Security Interest Act, 2002 read with proviso to Rule 8(6) of the Security Interest (Enforcement) Rules, 2002. Notice is hereby given to the public in general and in particular to the Borrowers and Guarantors that the below described immovable properties mortgaged to the Secured Creditor, the symbolic possession of which have been taken by the Authorized Officer of State Bank of India, the Secured Creditor, will be sold on “As is Where is”, “As is What is” and “Whatever there is” basis on 13.08.2020, for recovery of Rs. 22,86,38,334/- (Rupees Twenty Two Crores eighty six Lakhs Thirty Eight Thousand Three Hundred and Thirty Four Only) as on 30.06.2020 + interest due from 01.07.2020+Expenses there on due to the secured creditor from the borrowers i.e., 1) M/s Mangalagiri Textiles Private Limited and from the guarantors i.e. 1) Sri. Goli. Nagasaina Rao, 2) Sri.G.Rama Subramanyam, 3) Sri.Murugudu Lakshminarayana, 4) Sri.Mancha Vijaya Mohan Rao, 5) Sri.P.Adi Sudarsana Sundara Rao, 6) Sri.P.Sreedhar, 7) Sri.T.Sambasiva Rao, 8) Smt.Vangara Lakshmi Rajyam C/o M. Vijaya Mohana Rao. The reserve price, earnest money deposit particulars and short description of the properties with known encumbrances are mentioned below. PROPERTY NO.1: All that the Plant and Machinery belonging to the company M/s.Mangalagiri Textiles Pvt Ltd situated at Chinna Kakakani, Mangalagiri (M), Guntur District * The machinery to be lifted up by successful bidder immediately after payment of bid amount Reserve Price: Rs.7,56,00,000/- EMD: 75,60,000/- TIME:11:00 A.M. -
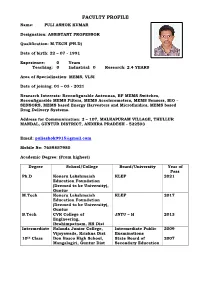
FACULTY PROFILE Name: PULI ASHOK KUMAR
FACULTY PROFILE Name: PULI ASHOK KUMAR Designation: ASSISTANT PROFESSOR Qualification: M.TECH (PH.D) Date of birth: 22 – 07 - 1991 Experience: 0 Years Teaching: 0 Industrial: 0 Research: 2.4 YEARS Area of Specialization: MEMS, VLSI Date of joining: 01 – 03 - 2021 Research Interests: Reconfigurable Antennas, RF MEMS Switches, Reconfigurable MEMS Filters, MEMS Accelerometers, MEMS Sensors, BIO – SENSORS, MEMS based Energy Harvesters and Microfluidics, MEMS based Drug Delivery Systems. Address for Communication: 2 – 107, MALKAPURAM VILLAGE, THULLUR MANDAL, GUNTUR DISTRICT, ANDHRA PRADESH - 522503 Email: [email protected] Mobile No: 7659857980 Academic Degree: (From highest) Degree School/College Board/University Year of Pass Ph.D Koneru Lakshmaiah KLEF 2021 Education Foundation (Deemed to be University), Guntur M.Tech Koneru Lakshmaiah KLEF 2017 Education Foundation (Deemed to be University), Guntur B.Tech CVR College of JNTU – H 2013 Engineering, Ibrahimpatnam, RR Dist Intermediate Nalanda Junior College, Intermediate Public 2009 Vijayawada, Krishna Dist Examinations 10th Class Don Bosco High School, State Board of 2007 Mangalagiri, Guntur Dist Secondary Education Experience particulars: (latest first) College/University Designation Duration Koneru Lakshmaiah SRF 1 year Education Foundation (Deemed to be University), Guntur Koneru Lakshmaiah JRF 1.4 Years Education Foundation (Deemed to be University), Guntur Administration: 00 Papers Published in referred Journals: 32 National: 00 International: 32 Papers presented in Conferences: -

List-Of-TO-STO-20200707191409.Pdf
Annual Review Report for the year 2018-19 Annexure 1.1 List of DTOs/ATOs/STOs in Andhra Pradesh (As referred to in para 1.1) Srikakulam District Vizianagaram District 1 DTO, Srikakulam 1 DTO, Vizianagaram 2 STO, Narasannapeta 2 STO, Bobbili 3 STO, Palakonda 3 STO, Gajapathinagaram 4 STO, Palasa 4 STO, Parvathipuram 5 STO, Ponduru 5 STO, Salur 6 STO, Rajam 6 STO, Srungavarapukota 7 STO, Sompeta 7 STO, Bhogapuram 8 STO, Tekkali 8 STO, Cheepurupalli 9 STO, Amudalavalasa 9 STO, Kothavalasa 10 STO, Itchapuram 10 STO, Kurupam 11 STO, Kotabommali 11 STO, Nellimarla 12 STO, Hiramandalam at Kothur 12 STO, Badangi at Therlam 13 STO, Pathapatnam 13 STO, Vizianagaram 14 STO, Srikakulam East Godavari District 15 STO, Ranasthalam 1 DTO, East Godavari Visakhapatnam District 2 STO, Alamuru 1 DTO, Visakhapatnam 3 STO, Amalapuram 2 STO, Anakapallli (E) 4 STO, Kakinada 3 STO, Bheemunipatnam 5 STO, Kothapeta 4 STO, Chodavaram 6 STO, Peddapuram 5 STO, Elamanchili 7 DTO, Rajahmundry 6 STO, Narsipatnam 8 STO, R.C.Puram 7 STO, Paderu 9 STO, Rampachodavaram 8 STO, Visakhapatnam 10 STO, Rayavaram 9 STO, Anakapalli(W) 11 STO, Razole 10 STO, Araku 12 STO, Addateegala 11 STO, Chintapalli 13 STO, Mummidivaram 12 STO, Kota Uratla 14 STO, Pithapuram 13 STO, Madugula 15 STO, Prathipadu 14 STO, Nakkapalli at Payakaraopeta 16 STO, Tuni West Godavari District 17 STO, Jaggampeta 1 DTO, West Godavari 18 STO, Korukonda 2 STO, Bhimavaram 19 STO, Anaparthy 3 STO, Chintalapudi 20 STO, Chintoor 4 STO, Gopalapuram Prakasam District 5 STO, Kovvur 1 ATO, Kandukuru 6 STO, Narasapuram -

Mangalagiri Mangalagiri
150 MANGALAGIRI ZONAL DEVELOPMENT PLAN PROPOSED LAND USE 2021 APPROVED BY THE GOVERNMENT VIDE G.O. MS NO:687 MA Dt.30-12-2006 LEGEND 230 220 ZONE BOUNDARY 210 CITY/TOWN BOUNDARY 190 200 WARD BOUNDARY LANDUSE BOUNDARY PROPOSED ROAD RAILWAY LINE 180 200 211/2 CANAL 190 PROPOSED BRIDGE MANDADAM 171 PLANNING ZONE 170 WATER BODY RESIDENTIAL 170 190 200 AGRICULTURAL AREA 171/2 180 COMMERCIAL 160 496 PUBLIC & SEMI PUBLIC 337 312 INDUSTRIAL 635 497 181 499 182 636 RECREATIONAL 498 638 336 BURIAL GROUND 500 537 634 186 313 335 501 538 637 316 183 TRANSPORT & COMMUNICATION 539 315 633 314 310 303 503 502 309 540 CONSERVATION 504 541 302 184 609 617 639 542 610 301 314 606/1 HIGH TENSION LINE 506 543 618 313 295 192 640 307 INDEX 546 544 613 632 308 304 300 615 642 306 P&G PARKS & PLAYGROUNDS 505 296 1 507 641 191 607 616 658 547 299 315 508 620 P PARKS 2 608 305 190 548 611 612 509 512 619 298 550 614 YERRABALEM LIFT IRRIGATION ROAD 631 660 297 552 189 210 510 630 317 511 621 643 670 672 549 657 188 60' 565 622 671 675 185 551 513 629 553 606 601 514 673 200 220 583 623 674 V.G.T.M URBAN DEVELOPMENT AUTHORITY 554 TO PENUMAKA 488 515 516 603 656 187 50 556 555 582 625 661 60 602 584 581 190 70 80 644 90 518 519 557 659 558 566 626 678 230 VIJAYAWADA 624 627 654 100 517 605 586 655 662 676 559 560 628 677 110 604 669 180 520 562 563 548 645 120 561 599 580 60' 585 697 698 563 567 564 653 VICE CHAIRMAN. -

Identify the Feasible Shallow Aquifer Zones in Coastal Sands in Part of Krishna Western Delta Region of A.P
International Journal of Applied Environmental Sciences ISSN 0973-6077 Volume 11, Number 2 (2016), pp. 535-540 © Research India Publications http://www.ripublication.com Identify the Feasible Shallow Aquifer Zones in Coastal Sands in Part of Krishna Western Delta Region of A.P. R. Venkata Ramana Research scholar, Department of Geology, Acharya Nagarjuna University and Assistant Professor, Department of civil Engineering, VFSTR University, Guntur district, Andhra Pradesh, India. Abstract The management of groundwater plays a vital role even inCostal zones. Costal sands in Guntur district occupies in an area of about 400 sq.km., covering Bapatla, Karlapalem, Pittalavanipalem, Nizampatnam, Cherukupally and Repallemandals. The groundwater in these coastal sands is being utilized through shallow open wells known as Doruvus. The Water table has been influenced by canal irrigation system and Krishna western delta and Precipitation. Fresh water in the coastal sands is floating above the saline ground water which occurs in the underlying clay layers. Thickness of sand aquifer is very limited in the area and varies from 2.5 to 5.0m. Occurrence of sand aquifer is not uniform and hence these coastal sandy areas with shallow fresh water in saline ground water region need better management technologies of Skimming and recharging to maintain environmental balance and sustainable irrigation. The paper discusses the distribution of costal sands and possibilities of management technology of skimming and recharging under Prevailing hydrogeological conditions. To assess the thickness of top sandy aquifers, subsurface lithological characteristics, detailed investigations were taken up in the study area. Keywords: Groundwater, Shallow aquifer zones, Coastal sands, Krishna Western delta, Andhra Pradesh INTRODUCTION The area covered with coastal sands consisting of mandals and lies in between East longitude from 80 25’25’00 to 80 50’00’ and North latitude from15 50’50’to 16 05’00’ as shown in the map(plate-1). -

APCRDA Region
LIST OF UN-AUTHORISED LAYOUTS IDENTIFIED BY APCRDA FROM THE YEAR 2008-NORTH ZONE VIJAYAWADA S.NO NAME OF THE OWNER/BUILDER VILLAGE & MANDAL S.R.No EXTENT IN A.C.'S REMARKS A.Kiran Kumar, S/o Rama Seshaiah, 1 Enikepadu 121/P, 122/P Ac. 3 Cents. 60-9-3, 6th line, Siddhartha Nagar, Vijayawada. 2 Not Known Enikepadu 121(P), 122(P) Ac. 3 Cents. 3 Not Known Enikepadu 54(P), 55(P) Ac. 1 Cents. 4 Not Known Enikepadu 121(P), 122(P) Ac. 3 Cents. 5 Vanka Anjaneyulu& his members Machavaram& Ibrahipatnam 15 Ac. 9.96 Cents 6 Sri Shaik Babavali Ibrahimpatnam 244/2A Ac.1.5 Cents 7 Sri. Ganne Venkatanarayana Prasad Gollapudi 533(P), 534(P) Ac.2.5 Cents 8 M. Sivanandam Gollapudi 601 Ac.1.96 Cents 9 Sri. Muvva Siva Nageswara Rao Gollapudi 601 Ac.1.04 Cents 10 Sri. B. Mallikharjuna Rao Gollapudi 73/5, 6 Ac.2.05 Cents 11 Sri. Janardhana Rao Guntupalli 236 Ac.2.95 Cents 12 Sri. Chigurupati Nageswara Rao Gollapudi 515/2, 525/2 Ac.3.05 Cents 13 Sri. Simhadri Rama Krishna Gollapudi 515/2, 525/2 Ac.1.95 Cents 14 Smt. Challguntla Padmaja Guntupalli 183/2 Ac.2.01 Cents 15 Sri Kotturu Ramesh Nunna 373 Ac. 2.63 Cents. 387/2, 3, 4, 16 T.Durga Prasad Nunna 390/1, 3, 397/1, Ac. 6.5 Cents. 2 17 B.Subba Rayadu Nunna 372/P Ac. 5 Cents. K.Subba Reddy 18 S/o. Veeraiah Nunna 868/P Ac. -

GOVERNMENT of ANDHRA PRADESH ABSTRACT Mines
GOVERNMENT OF ANDHRA PRADESH ABSTRACT Mines & Minerals – Reservation of Mineral Bearing areas located in RS No.221, Ananthavaram of Thullur Mandal, RS No. 484, Neerukonda, Bethapudi, RS No.202/1, Atmakuru Villages of Mangalagiri Mandal, RS No.47, 264, 94, 96, 224, 228, 215 etc. of Endroy & Lemalle Villages of Amaravathi Mandal, RS No.171 of Penumaka Village of Tadepalli Mandal exclusively in favour of AP Capital Region Development Authority (CRDA) for quarrying of Road Metal, Gravel, Granite Earth etc. under Rule 9-A (1) of APMMC Rules, 1966 - Orders - Issued. ---------------------------------------------------------------------------------------- INDUSTRIES & COMMERCE (M-II) DEPARTMENT G.O.Ms.No.177 Dated:16-12-2016 Read: From the DM&G, GoAP, Single File No. 174575/R3-3/2016, Dt.28.11.2016. --::o0o::-- ORDER : In the reference read above, the Director of Mines & Geology, Government of Andhra Pradesh has stated that the Assistant Director of Mines and Geology, Guntur has requested to reserve the Mineral Bearing areas located in RS No.221, Ananthavaram of Thullur Mandal, RS No. 484, Neerukonda, Bethapudi, RS No.202/1, Atmakuru Villages of Mangalagiri Mandal, RS No.47,264,94,96,224,228,215 etc. of Endroy & Lemalle Villages of Amaravathi Mandal, RS No.171 of Penumaka Village of Tadepalli Mandal, exclusively to AP Capital Region Development Authority (CRDA) for quarrying of Road Metal, Gravel, Granite Earth etc., for Capital constructions, under Rule 9-A (1) of APMMC Rules, 1966. 2. The Director of Mines and Geology, Government of Andhra Pradesh, Hyderabad has therefore requested the Government to reserve the above areas exclusively to AP Capital Region Development Authority (CRDA) for quarrying of Road Metal, Gravel, Granite Earth etc., for Capital constructions. -

SC&ST JA Total After Verification
LIST OF APPLICATIONS RECEIVED FOR THE POST OF JUNIOR ASSISTANT UNDER ST-G CATEGORY - 2020 Sl. Appli- Name of the Father/Husband Name & Address Date of Birth Age as on Qualifi- Total Marks Percen- Particulars of Addl. Caste Local / Remarks No cation Applicant as per SSC 30.11.2020 cation Marks Obtained tage Computer Qualifi- Certificate Non- No. ( years) in Degree of Marks Course cations Local 1 1 Ponnars Pavan S/o Ponnas Bheema Raju, D.No.13-1-317/8, 02.10.1994 27 B.Pharma CGPA10 8.65 86.50% NIL NIL ST LOCAL Kalyan Vinukonda Road Over Bridge Circle, Narasaraopet cy YERUKALA Mandal, Guntur District 2 2 Kelavathu Yesu S/o Kelavathu Saida Nai, D.No.2-15, Bhatrupalem 16.07.1996 25 BA 10 L-6.78,G- B- Grade MS.Office,DC NIL ST LOCAL Caste certificate Naik Village, Dachepalli Mandal, Guntur District-522414 6.81 A. not enclosed 3 3, 40, Kumbha S/o Kumbha Narasimha Rao, Velpuru Village, 08.07.1991 30 B.Com 0 L-3rd 0 NIL NIL ST LOCAL 120 Venkateswarlu Atachampet Mandal, Guntur District. class,G- YERUKALA Triple 3rd class 4 4 Manupati S/o M. Subba Rao, D.No.13-65/5/1/1, 02.06.1986 35 B.Com 1900 930 49 NIL MBA ST LOCAL Sudheer Seethanagaram, Tadepalli, Guntur - 522501 YERUKALA 5 5 Kundanapu S/o Kundanapu Sivaiah, D.No.5-44, Chintalabeedu, 21.07.1995 26 B.Sc 2400 1476 61.5 PGDCA M.Sc ST LOCAL Niranjan Rao Mathukumalli Village, Savalyapuram Mandal, YERUKALA Guntur District-522646. -

Mangalagiri Assembly Andhra Pradesh Factbook
Editor & Director Dr. R.K. Thukral Research Editor Dr. Shafeeq Rahman Compiled, Researched and Published by Datanet India Pvt. Ltd. D-100, 1st Floor, Okhla Industrial Area, Phase-I, New Delhi- 110020. Ph.: 91-11- 43580781, 26810964-65-66 Email : [email protected] Website : www.electionsinindia.com Online Book Store : www.datanetindia-ebooks.com Report No. : AFB/AP-087-0118 ISBN : 978-93-87415-61-4 First Edition : January, 2018 Third Updated Edition : June, 2019 Price : Rs. 11500/- US$ 310 © Datanet India Pvt. Ltd. All rights reserved. No part of this book may be reproduced, stored in a retrieval system or transmitted in any form or by any means, mechanical photocopying, photographing, scanning, recording or otherwise without the prior written permission of the publisher. Please refer to Disclaimer at page no. 158 for the use of this publication. Printed in India No. Particulars Page No. Introduction 1 Assembly Constituency at a Glance | Features of Assembly as per 1-2 Delimitation Commission of India (2008) Location and Political Maps 2 Location Map | Boundaries of Assembly Constituency in District | Boundaries 3-9 of Assembly Constituency under Parliamentary Constituency | Town & Village-wise Winner Parties- 2014-PE, 2014-AE, 2009-PE and 2009-AE Administrative Setup 3 District | Sub-district | Towns | Villages | Inhabited Villages | Uninhabited 10-12 Villages | Village Panchayat | Intermediate Panchayat Demographics 4 Population | Households | Rural/Urban Population | Towns and Villages by 13-15 Population Size | Sex Ratio -
Not Applicable for IOC/HPC
APPOINTMENT OF RETAIL OUTLET DEALERSHIPS IN AP BY IOC Location Sl. Name Of Location Revenue District Type of RO Estimated Category Type of Site Minimum Minimum Minimum Estimated Estimated Mode of Fixed Fee / Security No. (Not (Regular/Rur monthly (CC/DC/CFS) Frontage of Depth of Site Area of site working fund selection Min bid Deposit ( Rs applicable al) Sales Site (in M) (in M) (in Sq. M.). capital required for (Draw of amount ( Rs in Lakhs) for IOC/HPC) Potential requirement developmen Lots/Bidding in Lakhs) (MS+HSD) in for t of ) Kls operation of infrastructur RO (Rs in e at RO (Rs Lakhs) in Lakhs ) DRAW OF 1 BUKKAPATNAM VILLAGE & MANDAL ANANTAPUR Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS DRAW OF 2 GOTLUR VILLAGE, DHARMAVARAM MANDAL ANANTAPUR Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS DRAW OF 3 VAYALPADU (NOT ON NH - SH), VAYALAPADU MANDAL CHITTOOR Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS THONDAVADA VILLAGE (NOT ON NH/SH), CHANDRAGIRI DRAW OF 4 CHITTOOR Rural 48 SC CFS 20 20 400 0 0 0 2 MANDAL LOTS DRAW OF 5 DODDIPALLE (NOT ON NH/SH), PILERU MANDAL CHITTOOR Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS NARAYANA NELLORE VILLAGE (NOT ON SH/NH) NANDALUR DRAW OF 6 KADAPA Rural 48 SC CFS 20 20 400 0 0 0 2 MANDAL LOTS DRAW OF 7 ARAKATAVEMULA NOT ON SH/NH , RAJUPALEM MANDAL KADAPA Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS DRAW OF 8 GUTTURU VILLAGE, PENUKONDA MANDAL ANANTAPUR Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS DRAW OF 9 MADDALACHERUVU VILLAGE, KANAGANAPALLE MANDAL ANANTAPUR Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS DRAW OF 10 KALICHERLA (NOT ON NH/SH), PEDDAMANDYAM MANDAL CHITTOOR Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS CHINNACHEPALLE, NOT ON SH/ NH, KAMALAPURAM DRAW OF 11 KADAPA Rural 48 SC CFS 20 20 400 0 0 0 2 MANDAL LOTS DRAW OF 12 GUDIPADU NOT ON SH/NH, DUVVUR MANDAL KADAPA Rural 48 SC CFS 20 20 400 0 0 0 2 LOTS BUGGANIPALLE VILLAGE NOT ON NH/SH, BETHAMCHERLA DRAW OF 13 KURNOOL Rural 48 SC CFS 20 20 400 0 0 0 2 MANDAL LOTS DRAW OF 14 GOVINDPALLE VILLAGE NOT ON NH/SH, SIRVEL MANDAL KURNOOL Rural 48 ST CFS 20 20 400 0 0 0 2 LOTS DRAW OF 15 POLAKAL VILLAGE NOT ON NH/SH, C . -

Andhra Pradesh Government Officials. 1 S.K
CLIMARICE KICKOFF WORKSHOP AT GREAT LAKES CONFERENCE HALL, ICRISAT, PANTANCHERU. November 23, 2009 Andhra Pradesh Government Officials. 1 S.K. Joshi, IAS (chief Guest) 2 Sanjay Gupta, IFS Principal Secretary (Projects) Special Commissioner, PIM & RT Secretariat office, J-Block, Floor-7, Irrigation and Command Area Development Room No- 709, Hyderabad. Jalasoudha Building, Erramanzil Ph : 040-23453511, 23450666 Hyderabad-500083 E mail : [email protected] Ph: 040-23310945 Mobile: +91-9440418515 E mail: [email protected] National Institutions 3 Prof. B.N. Goswami 4 Dr. Hegde Director Centre for Planning, Monitoring and Evaluation Indian Institute of Tropical Meteorology National Institute of Rural Development(NIRD) Dr. Homi Bhabha Road, Pashan Rajendranagar, Hyderabad-500030,India Pune 411 008 5 Dr. VUM Rao, Principal Scientist (Ag. Met.) Central Research Institute for Dryland Agriculture (CRIDA) Santoshnagar, Saidabad P.O. Hyderabad - 500 059, Andhra Pradesh, INDIA Ph: +91-40-2453909 University Scientists from Hyderabad and Guntur districts: 6 Dr. M. Devender Reddy 7 Dr. K. Gurava Reddy Head, Water Technology Centre Scientist (Extension) Rajendranagar, Hyderabad Regional agricultural Research Station Ph: 040-24001445 Lam Farm E mail: [email protected] GUNTUR- 522 034 Ph : 0863 2524017, 9849484398 E mail : [email protected] 8 Dr. Yella Reddy Kaluvai 9 Dr. G. V. Subba Rao Principal Scientist & Project Manager Senior Scientist, Agronomy AP Water Management Project (APWMP) Andhra Pradesh Water Management Project First floor, College of Food science and (APWMP) technology First floor, College of Food science and Bapatla 522101, Guntur District technology, Mob: 9490490099 Bapatla, Guntur District Email: [email protected] 10 I. Rama Krishna Murthy Joint Director of Agriculture Collectorate compound, Guntur Ph: 0863-2234308 Fax: 0863-2234826 Mobile: 9849902748 ICRISAT 11 Sahrawat, Kanwar 12 Boomiraj , Kovilpillai ICRISAT, Patancheru ICRISAT, Patancheru 13 Dr. -

Identification of Groundwater Potential Zones Using Remote Sensing and GIS, Case Study: Mangalagiri Mandal
International Journal of Recent Technology and Engineering (IJRTE) ISSN: 2277-3878, Volume-7, Issue-6C2, April 2019 Identification of Groundwater Potential Zones using Remote Sensing and GIS, Case Study: Mangalagiri Mandal Kesana Sai Teja, Dinesh Singh Abstract: Ground water is one of the major sources that restricted aquifers. In India, over 90% of rural and almost contribute to the total annual supply. The explosive growth and 30% of urban population rely upon groundwater for meeting uneven distribution of population, poor irrigation practices, rapid their drinking and domestic prerequisites [4]. urbanization/industrialization, large-scale deforestation and Maps of ground water potential zones prepared from improper land use practices creates depletion of ground water. Therefore, increases demand of water for agriculture, household satellite images, serves as efficient tools for detailed ground and industry. The objective of this paper is to review techniques based hydro geological surveys which ultimately lead to the and methodologies applied for identifying groundwater potential selection of suitable sites for bore wells/Dug wells. Satellite zones using GIS and remote sensing. In order to evaluate the data offers the unique capability for extracting information ground water potential zones, different thematic maps such as on geology, drainage, land use/ land cover and soil from a geology, slope, soil, drainage density map, Land use and Land single image. Information on all these factors is essential in cover and surface water bodies at a 1: 50000 scale were prepared, using remotely-sensed data as well as topographical sheets and understanding the occurrence and movement of ground secondary data, collected from concern department.