Centaur Pharmaceuticals Pvt. Ltd. API Division - Ambernath Facility
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reg. No Name in Full Residential Address Gender Contact No
Reg. No Name in Full Residential Address Gender Contact No. Email id Remarks 20001 MUDKONDWAR SHRUTIKA HOSPITAL, TAHSIL Male 9420020369 [email protected] RENEWAL UP TO 26/04/2018 PRASHANT NAMDEORAO OFFICE ROAD, AT/P/TAL- GEORAI, 431127 BEED Maharashtra 20002 RADHIKA BABURAJ FLAT NO.10-E, ABAD MAINE Female 9886745848 / [email protected] RENEWAL UP TO 26/04/2018 PLAZA OPP.CMFRI, MARINE 8281300696 DRIVE, KOCHI, KERALA 682018 Kerela 20003 KULKARNI VAISHALI HARISH CHANDRA RESEARCH Female 0532 2274022 / [email protected] RENEWAL UP TO 26/04/2018 MADHUKAR INSTITUTE, CHHATNAG ROAD, 8874709114 JHUSI, ALLAHABAD 211019 ALLAHABAD Uttar Pradesh 20004 BICHU VAISHALI 6, KOLABA HOUSE, BPT OFFICENT Female 022 22182011 / NOT RENEW SHRIRANG QUARTERS, DUMYANE RD., 9819791683 COLABA 400005 MUMBAI Maharashtra 20005 DOSHI DOLLY MAHENDRA 7-A, PUTLIBAI BHAVAN, ZAVER Female 9892399719 [email protected] RENEWAL UP TO 26/04/2018 ROAD, MULUND (W) 400080 MUMBAI Maharashtra 20006 PRABHU SAYALI GAJANAN F1,CHINTAMANI PLAZA, KUDAL Female 02362 223223 / [email protected] RENEWAL UP TO 26/04/2018 OPP POLICE STATION,MAIN ROAD 9422434365 KUDAL 416520 SINDHUDURG Maharashtra 20007 RUKADIKAR WAHEEDA 385/B, ALISHAN BUILDING, Female 9890346988 DR.NAUSHAD.INAMDAR@GMA RENEWAL UP TO 26/04/2018 BABASAHEB MHAISAL VES, PANCHIL NAGAR, IL.COM MEHDHE PLOT- 13, MIRAJ 416410 SANGLI Maharashtra 20008 GHORPADE TEJAL A-7 / A-8, SHIVSHAKTI APT., Male 02312650525 / NOT RENEW CHANDRAHAS GIANT HOUSE, SARLAKSHAN 9226377667 PARK KOLHAPUR Maharashtra 20009 JAIN MAMTA -
SR NO First Name Middle Name Last Name Address Pincode Folio
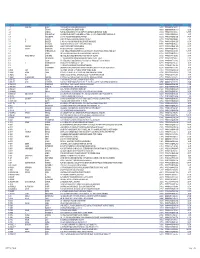
SR NO First Name Middle Name Last Name Address Pincode Folio Amount 1 A SPRAKASH REDDY 25 A D REGIMENT C/O 56 APO AMBALA CANTT 133001 0000IN30047642435822 22.50 2 A THYAGRAJ 19 JAYA CHEDANAGAR CHEMBUR MUMBAI 400089 0000000000VQA0017773 135.00 3 A SRINIVAS FLAT NO 305 BUILDING NO 30 VSNL STAFF QTRS OSHIWARA JOGESHWARI MUMBAI 400102 0000IN30047641828243 1,800.00 4 A PURUSHOTHAM C/O SREE KRISHNA MURTY & SON MEDICAL STORES 9 10 32 D S TEMPLE STREET WARANGAL AP 506002 0000IN30102220028476 90.00 5 A VASUNDHARA 29-19-70 II FLR DORNAKAL ROAD VIJAYAWADA 520002 0000000000VQA0034395 405.00 6 A H SRINIVAS H NO 2-220, NEAR S B H, MADHURANAGAR, KAKINADA, 533004 0000IN30226910944446 112.50 7 A R BASHEER D. NO. 10-24-1038 JUMMA MASJID ROAD, BUNDER MANGALORE 575001 0000000000VQA0032687 135.00 8 A NATARAJAN ANUGRAHA 9 SUBADRAL STREET TRIPLICANE CHENNAI 600005 0000000000VQA0042317 135.00 9 A GAYATHRI BHASKARAAN 48/B16 GIRIAPPA ROAD T NAGAR CHENNAI 600017 0000000000VQA0041978 135.00 10 A VATSALA BHASKARAN 48/B16 GIRIAPPA ROAD T NAGAR CHENNAI 600017 0000000000VQA0041977 135.00 11 A DHEENADAYALAN 14 AND 15 BALASUBRAMANI STREET GAJAVINAYAGA CITY, VENKATAPURAM CHENNAI, TAMILNADU 600053 0000IN30154914678295 1,350.00 12 A AYINAN NO 34 JEEVANANDAM STREET VINAYAKAPURAM AMBATTUR CHENNAI 600053 0000000000VQA0042517 135.00 13 A RAJASHANMUGA SUNDARAM NO 5 THELUNGU STREET ORATHANADU POST AND TK THANJAVUR 614625 0000IN30177414782892 180.00 14 A PALANICHAMY 1 / 28B ANNA COLONY KONAR CHATRAM MALLIYAMPATTU POST TRICHY 620102 0000IN30108022454737 112.50 15 A Vasanthi W/o G -

Thane District NSR & DIT Kits 15.10.2016
Thane district UID Aadhar Kit Information SNO EA District Taluka MCORP / BDO Operator-1 Operator_id Operator-1 Present address VLE VLE Name Name Name Mobile where machine Name Mobile number working (only For PEC) number 1 Abha System and Thane Ambarnath BDO abha_akashS 7507463709 /9321285540 prithvi enterpriss defence colony ambernath east Akash Suraj Gupta 7507463709 Consultancy AMBARNATH thane 421502 Maharastra /9321285540 2 Abha System and Thane Ambarnath BDO abha_abhisk 8689886830 At new newali Nalea near pundlile Abhishek Sharma 8689886830 Consultancy AMBARNATH Maharastraatre school, post-mangrul, Telulea, Ambernath. Thane,Maharastra-421502 3 Abha System and Thane Ambarnath BDO abha_sashyam 9158422335 Plot No.901 Trivevi bhavan, Defence Colony near Rakesh Sashyam GUPta 9158422335 Consultancy AMBARNATH Ayyappa temple, Ambernath, Thane, Maharastra- 421502 4 Abha System and Thane Ambarnath BDO abha_pandey 9820270413 Agrawal Travels NL/11/02, sector-11 ear Sandeep Pandey 9820270413 Consultancy AMBARNATH Ambamata mumbai, Thane,Maharastra-400706 5 Abha System and Thane Ambarnath BDO pahal_abhs 8689886830 Shree swami samath Entreprises nevalinaka, Abhishek Sharma 8689886830 Consultancy AMBARNATH mangrul, Ambarnath, Thane,Maharastra-421301 6 Vakrangee LTD Thane Ambarnath BDO VLE_MH610_NS055808 9637755100/8422883379 Shop No.1, Behind Datta Mandir Durga Devi Pada Priyanka Wadekar 9637755100/ AMBARNATH /VLE_MCR610_NS073201 Old Ambernath, East 421501 8422883379 7 Vakrangee LTD Thane Ambarnath BDO VLE_MH610_NS076230 9324034090 / Aries Apt. Shop No. 3, Behind Bethel Church, Prashant Shamrao Patil 9324034090 / AMBARNATH 8693023777 Panvelkar Campus Road, Ambernath West, 8693023777 421505 8 Vakrangee LTD Thane Ambarnath BDO VLE_MH610_NS086671 9960261090 Shop No. 32, Building No. 1/E, Matoshree Nagar, Babu Narsappa Boske 9960261090 AMBARNATH Ambarnath West - 421501 9 Vakrangee LTD Thane Ambarnath BDO VLE_MH610_NS037707 9702186854 House No. -

Biodiversity of Fungi from Soil and Water Samples from Waldhuni River Deepak Pardeshi and Sharda Vaidya*
ISSN: 2347-3215 Volume 3 Number 4 (April-2015) pp. 190-195 www.ijcrar.com Biodiversity of fungi from soil and water samples from Waldhuni River Deepak Pardeshi and Sharda Vaidya* Smt. C. H. M. College, Ulhasnagar-03, India *Corresponding author KEYWORDS A B S T R A C T Waldhuni Waldhuni is a small river originating at Kakole Lake near Ambarnath and River, unites with Ulhas River near Kalyan with its total length of 31.8 Km. The PDA, river is so much polluted that it is now referred to as Waldhuni Nallah. The Fungi soil and water samples (surface and deep water) were collected frequently from four locations of the river. The soil samples were dried and used for culturing. The water samples were used immediately for culturing. The medium used for culturing all the samples was Potato dextrose Agar. A number of fungi were isolated such as Trichoderma, Aspergillus, Penicillium, Auriobasidium, etc. Introduction River Waldhuni is a left bank tributary of Freshwater fungi are a diverse and Ulhas River. The Waldhuni River flows heterogeneous group, comprising many through Ambernath, Ulhasnagar and Kalyan species from different orders in which and its entire stretch is 31.8 Km. The river Ascomycetes and Hyphomycetes are bank has maximum encroachment from dominant orders. Ulhasnagar on the east and Ashoknagar and Shivajinagar in Kalyan on the west of its Fresh water Hyphomycetes were practically bank.). It flows through thickly populated untouched by the pioneering work of who area, which was severely polluted due to recognized them, as Aquatic domestic and industrial sewage. -
Aadhaar Card Enrollment Centers List
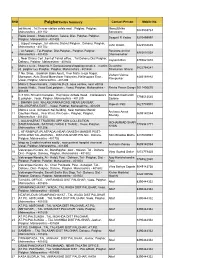
SNO PalgharCentre Summary Contact Person Mobile No. sai bhumi , 1st flr near station safale east , Palghar, Palghar, Manoj Bhika 1 9823533764 Maharashtra - 401102 Sonawane Raids Arced, , Shop 24 Manor, Taluka, Dist- Palghar, Palghar, 2 Swapnil R Yadav 9270499997 Palghar, Maharashtra - 401403 ., at/post vangaon,, tal- dahanu District Palghar , Dahanu, Palghar, 3 Jahir shaikh 9423533665 Maharashtra - 401702 ., At Satpati, , Tal-Palghar, Dist-Palghar-, Palghar, Palghar, Ravindra Arvind 4 8983691038 Maharashtra - 401405 Dharmameher ., Near Dhanu Cort ,front of Tahisil office, , Tal Dahanu Dist Palghar, 5 Jayvanti Bari 8793621678 Dahanu, Palghar, Maharashtra - 401602 Maha e seva , Shop No.9, Dariyasarang shopping complex, , mahim Devendra 6 9822764241 rd, palghar (w), Palghar, Palghar, Maharashtra - 401404 Shantaram Gharat 1 No. Shop, , Badshah Baba Apart., Veer Mata Jeejai Nagar, Vishant Vishnu 7 Moregaon, Auto Stand Moreshwar Vidayalay, Nallasopara East , 8446168882 Rampurkar Vasai, Palghar, Maharashtra - 401209 Maha E Sewa Kendra, , Gala No.B-23, aqsa comlex, near vithhal 8 mandir,Waliv,, Vasai East,palghar -, Vasai, Palghar, Maharashtra - Rekha Pravin Dange 9011490670 401208 C-7 Shiv Shrushti Complex , Raj Nagar,Achole Road, , Nallasopara Santosh Dasharath 9 7798513545 E palghar-, Vasai, Palghar, Maharashtra - 401209 Zadane ., DHANIV GAV, NALASOPARA ROAD, NEAR SAN BAR, , 10 Rupesh Patil 9627739991 NALASOPARA EAST. , Vasai, Palghar, Maharashtra - 401209 Maha e seva, Icchapurti Sai Building, Near Saibaba Mandir, Archana Amrut 11 Gaothan Road, -

BOMBAY Story of the Island City
BOMBAY Story of the Island City By A. D. PUSALKER & V. G. DIGHE -~INDIA ORIENTAL CONFERENCE BOMBAY. 1949 BOMBAY Story <:>f the Island-City. By A. D. PUSALKER & V. G. DIGHE ALL INDIA OltiEN'l'AL CONFERENCE BOMBAY. 1 9 .4 9 Printed bJ G. G. Patbue at 'l'be Popular Pna (Bom.) Ltd., ....~ 7 Uld Publlabed .., the Local s-.r,., All Jndla OrieDtal Confennce, Town Hall, Bombay 1. PRICE IUIPBES '!:. PREFACE The rise and growth of Bombay present interesting problems to a student of history. While the city has been built in comparatively modern times the formation of the island and its rock temples arouse the interest of the geologist and the antiquarian. The history of the island upto 1500 A.D. is not very eventful; this tropical island and its native population slumbered in peaceful repose till the first European set foot on its soil and set in train forces which transformed it into one of the largest cities in the East and made it the beehive of commerce and industry. How this transformation was wrought, what factors contributed to it, has been narrated in the pages that follow. The object of the book as the title explains is to narrate the story of the island city in simple outline. The main sources of information are Edwardes' Rise of Bombay and the statistical Account of the town and island of Bombay based on old Government records and prepared for the Bombay Gazetteer. Other sources have also been consulted. The account of research institutes in the city will, it is hoped, interest Orientalists and Historians. -

Maharashtra State Boatd of Sec & H.Sec Education Pune
MAHARASHTRA STATE BOATD OF SEC & H.SEC EDUCATION PUNE PAGE : 1 College wise performance ofFresh Regular candidates for HSC MARCH-2019 Candidates passed College No. Name of the collegeStream Candidates Candidates Total Pass Registerd Appeared Pass UDISE No. Distin- Grade Grade Pass Percent ction I II Grade 16.01.001 RAYATE VIBHAG HIGH SCHOOL & JUNIOR COLLEGE, ARTS 124 124 0 21 68 1 90 72.58 27210501802 RAYATE TOTAL 124 124 0 21 68 1 90 72.58 16.01.002 SAKARAM SHETH JR.COLL, KATAI-KOLE, ARTS 50 50 3 18 25 0 46 92.00 27210508002 TQ.KALYAN,THANE TOTAL 50 50 3 18 25 0 46 92.00 16.01.003 KAKADPADA VIBHAG MADH VA UCHCHA MADH VIDY ARTS 31 31 0 4 20 1 25 80.64 27210503602 KAKADPDA TOTAL 31 31 0 4 20 1 25 80.64 16.01.004 ARTS COMM & SCI JR COLL GOVELI,PO RAYATE SCIENCE 146 146 2 39 99 0 140 95.89 27210501307 421301 ARTS 128 128 0 22 87 1 110 85.93 COMMERCE 211 211 1 30 132 28 191 90.52 TOTAL 485 485 3 91 318 29 441 90.92 16.01.005 BHARTIYA SAINIKI VID.& SCIENCE 30 30 1 2 25 2 30 100.00 27210503707 JR.COLLEGE,KHADAWALI,KALYAN TOTAL 30 30 1 2 25 2 30 100.00 16.01.006 MAHAVIR HIGH SCHOOL & ARTS 5 5 0 0 0 2 2 40.00 27210506316 JR.COLLEGE,MHARALGAON,KALYAN TOTAL 5 5 0 0 0 2 2 40.00 16.01.007 G.K.S. -

Thane City District – Maharashtra
Thane City District – Maharashtra Sl. Name of the Complete postal E-mail No. Sub-Division address of ACP/Dy. Telephone Numbers along with STD and Police SP/SDPO office and Code Station Police Station along with PIN Code Office Residence Fax 1. ACP Thane Talaopali, Above 022- Nil Nil Nil Division Perfect Motor 25343365 Driving Training School, Thane- 400601, Maharashtra 2. ACP Wagle Talaopali, Above 022- Nil Nil Nil Estate Division Perfect Motor 25341924 Driving Training School, Thane- 400601, Maharashtra 3. ACP Kalwa Near Central Jail, 022- Nil Nil Nil Division Thane-400601, 25344322 Maharashtra 4. ACP Bhiwandi Near Prant Office, 02522- Nil Nil Nil (E) Division Bhiwandi, Dist. 250650 Thane-421302, Maharashtra 5. ACP Bhiwandi Near J.M.F.C. Court 02522- Nil Nil Nil (W) Division Bhiwandi, Dist. 252650 Thane-421302, Maharashtra 6. ACP Kalyan Alishan Tower, 0251- Nil Nil acpkalian@thanepo Division Murbad Road, 2315043 lice.org Kalyan, Dist Thane- 421301, Maharashtra 7. ACP Dombivli Above Ramnagar PS, 0251- Nil Nil Nil Division Dombivli (E), 2861011 Thane-421201, Maharashtra 8. ACP Ulhasnagar Pawai Chowk, 0251- Nil Nil Nil Division Ulhasnagar – 3. Dist 2569076 Thane-421003, Maharashtra 9. ACP Near Panchayat 0251- Nil Nil Nil Ambarenath Samiti office, 2682940 Division Ambernath (W), Dist Thane-421501, Maharashtra 10. Thane Nagar PS Tahasildar 022- Nil 022- sr.pithanenagarps Compound, Station 25333416 25333416 @thanepolice.org Road, Thane-400601, Maharashtra 11. Kopri PS Dinesh 022- Nil 022- sr.pikoprips@thane C.Op.H.Society 25323800 25323800 police.org Thane-400603, Maharashtra 12. Naupada PS M.G.Road, Thane- 022- Nil 022- Nil 400602, Maharashtra 25423300 25423300 13. -
KALYAN Working Name of CSC Design SL.NO
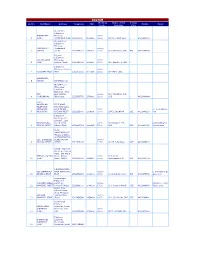
KALYAN Working Name of CSC Design SL.NO. CSC Name Address Telephone FAX Mobile Email Hours Incharge ation Ambarnath Telephone AMBARNATH Exchange 08.00 - 1 (EAST) ,Ambarnath (East) 251260400 2603020 20.00 Shri B V Wagh SDE 0 9423985688 Ground Floor Telephone Exchange AMBARNATH ,Ambarnath 08.00 - 2 (WEST) (west) 2512685010 2600601 20.00 Smt Swetarani ,JTO JTO 9423985698 Kulgaon Telephone CSC KULGAON Exchange, 08.00 - 3 (EAST) Kulgaon (East) 2512690197 2690197 20.00 Shri Shaikh I A, SDE 0 Telephone Exchange Kulgaon 08.00 - 4 KULGAON (WEST) West 2512670010 2677196 20.00 Shri Manji ,SDE 0 AMBARNATH 5 (WEST) REPEATED CSC Ground Floor , Ulhasnagar Telephone Exchange , Near CSC Goal Maidan, 08.00 - Shri Chaudhary N.K. 6 ULHASNAGAR Ulhasnagar. 2512565758 2566161 20.00 SDE 0 9423984949 C.S.C. KALATALAO TELEPHONE TELEPHONE EXCHANE BLDG EXCHANGE, KALA TALAO, 08.00 - [email protected] 7 KALYAN(W) KALYAN (WEST) 2512315800 2319090 20.00 SHRI C.N.SONAR SDE 9423986250 o.in Khadakpada Telephone Exchange, R J Complex , Birla KHADAKPADA, College Road 09.30 - Shri Dhayade P.H. prakashdhayade 8 KALYAN (WEST) Kalyan (West) 2512231111 2230195 17.00 SDE SDE 9423984066 @gmail.com NEAR MAHATMAPHULE POLICE CHOUKI, ADMINISTRATIVE CSC, CTO BLDG, BLDG, KALYAN 08.00 - 9 KALYAN (WEST) (WEST) 2512315188 17.30 Shri M.P.Ashtekar SDE 9423986600 Netivli Telephone Exchange, Suchak Naka , Shil Phata NETIVLI, KALYAN Road , Kalyan 09.30 - Smt Sunita 10 (EAST) (East) 421306 2512356168 2351188 17.00 Pachkawade JTO JTO 9422131783 DOMBIVALI MIDC CSC DOMBIVALI AREA DOMBIVALI 08.00 - p_deshmykh1@y 11 [E] MIDC EXCH EAST 2512452958 2802300 17.30 Shri.P.B.Deshmukh JTO 9423985566 ahoo.com Vishnunagar Telephone VISHNOO NAGAR, Exchange, 08.00 - jayashree_murli 12 DOMBIVLI (WEST) Dombivli (West) 2512492000 2489000 17.30 Smt Jayashri Murali SDE 9423989988 @bsnl.co.in Omkar Bldg. -

SIEMENS LIMITED List of Outstanding Warrants As on 18Th March, 2020 (Payment Date:- 14Th February, 2020) Sr No
SIEMENS LIMITED List of outstanding warrants as on 18th March, 2020 (Payment date:- 14th February, 2020) Sr No. First Name Middle Name Last Name Address Pincode Folio Amount 1 A P RAJALAKSHMY A-6 VARUN I RAHEJA TOWNSHIP MALAD EAST MUMBAI 400097 A0004682 49.00 2 A RAJENDRAN B-4, KUMARAGURU FLATS 12, SIVAKAMIPURAM 4TH STREET, TIRUVANMIYUR CHENNAI 600041 1203690000017100 56.00 3 A G MANJULA 619 J II BLOCK RAJAJINAGAR BANGALORE 560010 A6000651 70.00 4 A GEORGE NO.35, SNEHA, 2ND CROSS, 2ND MAIN, CAMBRIDGE LAYOUT EXTENSION, ULSOOR, BANGALORE 560008 IN30023912036499 70.00 5 A GEORGE NO.263 MURPHY TOWN ULSOOR BANGALORE 560008 A6000604 70.00 6 A JAGADEESWARAN 37A TATABAD STREET NO 7 COIMBATORE COIMBATORE 641012 IN30108022118859 70.00 7 A PADMAJA G44 MADHURA NAGAR COLONY YOUSUFGUDA HYDERABAD 500037 A0005290 70.00 8 A RAJAGOPAL 260/4 10TH K M HOSUR ROAD BOMMANAHALLI BANGALORE 560068 A6000603 70.00 9 A G HARIKRISHNAN 'GOKULUM' 62 STJOHNS ROAD BANGALORE 560042 A6000410 140.00 10 A NARAYANASWAMY NO: 60 3RD CROSS CUBBON PET BANGALORE 560002 A6000582 140.00 11 A RAMESH KUMAR 10 VELLALAR STREET VALAYALKARA STREET KARUR 639001 IN30039413174239 140.00 12 A SUDHEENDHRA NO.68 5TH CROSS N.R.COLONY. BANGALORE 560019 A6000451 140.00 13 A THILAKACHAR NO.6275TH CROSS 1ST STAGE 2ND BLOCK BANASANKARI BANGALORE 560050 A6000418 140.00 14 A YUVARAJ # 18 5TH CROSS V G S LAYOUT EJIPURA BANGALORE 560047 A6000426 140.00 15 A KRISHNA MURTHY # 411 AMRUTH NAGAR ANDHRA MUNIAPPA LAYOUT CHELEKERE KALYAN NAGAR POST BANGALORE 560043 A6000358 210.00 16 A MANI NO 12 ANANDHI NILAYAM -

WESTERN ZONE) BENCH, PUNE APPLICATION No. 37/2013 (WZ
BEFORE THE NATIONAL GREEN TRIBUNAL (WESTERN ZONE) BENCH, PUNE APPLICATION No. 37/2013 (WZ) CORAM: Hon’ble Mr. Justice V.R. Kingaonkar (Judicial Member) Hon’ble Dr. Ajay A. Deshpande (Expert Member) B E T W E E N: 1. Vanashakti Public Trust, Unique Industrial Estate, Twin Tower Lane, Prabhadevi, Mumbai-400 025 2. Stalin Dayanand, Aged 48 yrs. Director of Vanashakti, Having its office at Unique Industrial Estate, Twin Tower Lane, Prabhadevi, Mumbai 400 025 ….Applicants A N D 1. Maharashtra Pollution Control Board, Through Its Member Secretary, Kalpataru Building, Sion, Mumbai – 22 2. The Maharashtra State Environment Department, Through Its Principal Secretary, Having its office at Mantralaya, Churchgate, Mumbai 400 032 (J) Application No.37/2013 (WZ) 1 3. Union of India, Through Secretary, Ministry of Environment & Forests, Paryavaran Bhavan, Lodi Road, New Delhi. 4. Central Pollution Control Board, Parivesh Bhawan, CBD-cum- Office Complex, East Arjun Nagar, Delhi 110 032. 5. The Municipal Commissioner, Kalyan Dombivili Municipal Corporation, Having its office at Shankarrao Chowk, Kalyan (West), Distt : Thane 6. The Municipal Commissioner, Ulhasnagar Municipal Corporation, Having its office of UMC Headquarters, Ulhasnagar, Distt : Thane. 7. The President, Ambarnath Municipal Council, Having its office at Gandhi Chowk, Ambarnath, Distt : Thane 8. Maharashtra Industrial Development Corporation (MIDC), Office at Mahakali Caves road, Andheri East, Mumbai 400 003. …Respondents Counsel for Appellant : Mrs. Gayatri Singh, Adv. a/w. Mr. Stalin D. Counsel for Respondent No. 1 & 2 : Mr. Rajendra Raghuwanshi, Adv. (J) Application No.37/2013 (WZ) 2 Mr. D.M. Gupte, Adv. Mrs. Supriya Dangare, Advs. Counsel for Respondent No.4 : Mrs. -

1 Maharashtra Regional and Town Planning Act, 1966. Modification To
Maharashtra Regional and Town Planning Act, 1966. Modification to Development Control Regulations for Municipal Corporation's of Thane,Kalyan-Dombivali,Mira-Bhayander,B hiwandi-Nizampur, and Special Planning Authority areas at Vasai-Virar subregion and Ambernath, Kulgaon, Badlapur & surrounding notified area, and Municipal Council of Panvel under section 37(1) read with section 154 of the said Act. GOVERNMENT OF MAHARASHTRA Urban Development Department Mantralaya, Mumbai 400032. Dated the 4th November 2008. ORDER No. TPS –1208/MMR/CR- 393/08/UD-12 Whereas the Development Plan and Development Control Regulations (hereinafter referred to as “the said Regulations”) for Municipal Corporation's of Thane,Kalyan-Dombivali,Mira-Bhayander,Bhiwandi-Nizampur, and Special Planning Authority areas at Vasai-Virar subregion and Ambernath, Kulgaon, Badlapur & surrounding notified area, and Municipal Council of Panvel (hereinafter referred to as “the said authority”) within Mumbai Metropolitan Region have been sanctioned by Government in Urban Development Department, under section 31(1) of the Maharashtra Regional and Town Planning Act, 1966 (hereinafter referred to as “the said Act”) from time to time and they have come into force , And whereas Govt. of Maharashtra has formulated the Housing Policy for the State of Maharashtra and the main objective of this policy is to provide the affordable houses for poor on rental basis. And whereas Mumbai Metropolitan Region Development Authority (MMRDA) has formulated a proposal to built Rental houses under different models within Mumbai Metropolitan Region(MMR) And whereas MMRDA vide its letter dated 21/6/08 and 29/7/08 has requested to carry out required modifications in Development Control Regulations 1 and to appoint MMRDA as Project Implementing Agency for all rental housing projects undertaken in Mumbai Metropolitan Region by constructing or procuring constructed self contained dwelling units of 160 sq.ft.