Seroprevalence of Toxoplasma Gondii and Associated Hematological Alterations in Small Ruminants of D.G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Critical Discourse Analysis of Marsiya-E-Hussain
Religious Ideology and Discourse: A Critical Discourse Analysis of Marsiya-e-Hussain Snobra Rizwan Lecturer, Department of English Bahauddin Zakariya University, Multan Pakistan Tariq Saeed Assisstant Professor, Department of English Bahauddin Zakariya University, Multan Pakistan Ramna Fayyaz Lecturer, Department of English Bahauddin Zakariya University, Multan Pakistan ABSTRACT This paper employs Fairclough’s framework of critical discourse analysis (Fairclough, 2001; 2003) as a research tool to demonstrate how mourning discourse of marsiya manages to win favourite responses from Pakistani audiences by foregrounding certain linguistic conventions. The data comprising popular marsiyas are based on responses obtained through a small-scale survey and are analyzed from the perspective of ideology and emotive appeal embedded in discourse. The analysis illustrates that discourse conventions of marsiya—in addition to traditional commemoration of martyrdom of Imam Hussian—serve to elaborate, explain and disseminate religious doctrines in Pakistani Shi‘ah masses. Keywords: Marsiya, Critical Discourse Analysis, Ideology, Shiism 1. Introduction This paper provides a close study to examine the distinguishing features of marsiya-e-Hussain and the way discursive choices of certain transitivity features, figurative language and lyrical conventions serve to make it a distinct poetic genre of its own. Though marsiya recitation is taken to be a means of commemorating the martyrdom of Imam Hussain; nevertheless, it means much more to Shi’ia community. Along with other mourning rituals, marsiya is considered to be a means of seeking waseela (mediation) from the saints, teaching and learning religious ideologies, seeking God’s pleasure and so on (‘Azadari; mourning for Imam Hussain’, 2009). All these objectives are achieved by following certain discourse conventions which in turn construct certain discursive reality and weigh heavily on the formation of distinctive opinion and religious ideology in Shi‘ah masses. -
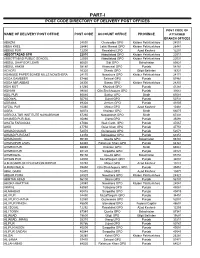
Part-I: Post Code Directory of Delivery Post Offices
PART-I POST CODE DIRECTORY OF DELIVERY POST OFFICES POST CODE OF NAME OF DELIVERY POST OFFICE POST CODE ACCOUNT OFFICE PROVINCE ATTACHED BRANCH OFFICES ABAZAI 24550 Charsadda GPO Khyber Pakhtunkhwa 24551 ABBA KHEL 28440 Lakki Marwat GPO Khyber Pakhtunkhwa 28441 ABBAS PUR 12200 Rawalakot GPO Azad Kashmir 12201 ABBOTTABAD GPO 22010 Abbottabad GPO Khyber Pakhtunkhwa 22011 ABBOTTABAD PUBLIC SCHOOL 22030 Abbottabad GPO Khyber Pakhtunkhwa 22031 ABDUL GHAFOOR LEHRI 80820 Sibi GPO Balochistan 80821 ABDUL HAKIM 58180 Khanewal GPO Punjab 58181 ACHORI 16320 Skardu GPO Gilgit Baltistan 16321 ADAMJEE PAPER BOARD MILLS NOWSHERA 24170 Nowshera GPO Khyber Pakhtunkhwa 24171 ADDA GAMBEER 57460 Sahiwal GPO Punjab 57461 ADDA MIR ABBAS 28300 Bannu GPO Khyber Pakhtunkhwa 28301 ADHI KOT 41260 Khushab GPO Punjab 41261 ADHIAN 39060 Qila Sheikhupura GPO Punjab 39061 ADIL PUR 65080 Sukkur GPO Sindh 65081 ADOWAL 50730 Gujrat GPO Punjab 50731 ADRANA 49304 Jhelum GPO Punjab 49305 AFZAL PUR 10360 Mirpur GPO Azad Kashmir 10361 AGRA 66074 Khairpur GPO Sindh 66075 AGRICULTUR INSTITUTE NAWABSHAH 67230 Nawabshah GPO Sindh 67231 AHAMED PUR SIAL 35090 Jhang GPO Punjab 35091 AHATA FAROOQIA 47066 Wah Cantt. GPO Punjab 47067 AHDI 47750 Gujar Khan GPO Punjab 47751 AHMAD NAGAR 52070 Gujranwala GPO Punjab 52071 AHMAD PUR EAST 63350 Bahawalpur GPO Punjab 63351 AHMADOON 96100 Quetta GPO Balochistan 96101 AHMADPUR LAMA 64380 Rahimyar Khan GPO Punjab 64381 AHMED PUR 66040 Khairpur GPO Sindh 66041 AHMED PUR 40120 Sargodha GPO Punjab 40121 AHMEDWAL 95150 Quetta GPO Balochistan 95151 -

Internet Use by Social Scientists at the Bahauddin Zakariya University, Multan, Pakistan: a Survey
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UNL | Libraries University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Library Philosophy and Practice (e-journal) Libraries at University of Nebraska-Lincoln 11-2011 Internet Use by Social Scientists at the Bahauddin Zakariya University, Multan, Pakistan: A Survey Rubina Bhatti Islamia University of Bahawalpur, [email protected] Mahe Bushra Asghar The Islamia University of Bahawalpur South Punjab, Pakistan Sarwat Mukhtar The Islamia University of Bahawalpur South Punjab, Pakistan Tariq M. Chohan The Islamia University of Bahawalpur South Punjab, Pakistan Follow this and additional works at: https://digitalcommons.unl.edu/libphilprac Part of the Library and Information Science Commons Bhatti, Rubina; Bushra Asghar, Mahe; Mukhtar, Sarwat; and Chohan, Tariq M., "Internet Use by Social Scientists at the Bahauddin Zakariya University, Multan, Pakistan: A Survey" (2011). Library Philosophy and Practice (e-journal). 667. https://digitalcommons.unl.edu/libphilprac/667 http://unllib.unl.edu/LPP/ Library Philosophy and Practice 2011 ISSN 1522-0222 Internet Use by Social Scientists at the Bahauddin Zakariya University, Multan, Pakistan: A Survey Dr. Rubina Bhatti Assistant Professor & Coordinator, M.Phil Programme Department of Library and information Science The Islamia University of Bahawalpur South Punjab, Pakistan Mrs. Mahe Bushra Asghar Lecturer Department of Library and information Science The Islamia University of Bahawalpur South Punjab, Pakistan Mrs. Sarwat Mukhtar Lecturer Department of Library and information Science The Islamia University of Bahawalpur South Punjab, Pakistan Tariq M. Chohan Librarian The Islamia University Library of Bahawalpur The Islamia University of Bahawalpur South Punjab, Pakistan Introduction Bahauddin Zakariya University Multan South Punjab, Pakistan Bahauddin Zakariya University Multan was established in 1975 by an act of the Punjab Legislative Assembly. -

Department of History and Culture Faculty of Humanities and Languages Jamia Millia Islamia, New Delhi
Department of History and Culture Faculty of Humanities and Languages Jamia Millia Islamia, New Delhi Invites you to a lecture By Yogesh Snehi On Sufi Shrines in Post-Partition Punjab? Dreams, Memory and Continuities 18 October, 2012 (Thursday) 12:15 PM Venue: Seminar Room, Department of History and Culture, JMI Prof. Syed Hasan Mahmud, Department of History and Culture will chair the session Medieval Punjab was among the first regions of South Asia to experience the significant influence of early Sufi mystics. The early sufi orders which found their presence in the region included the Chishtis and Suhrawardis in the Sultanate milieu, and Nasqbandis and Qadiris in the Mughal period. By the end of the Mughal empire, the province was dotted with the shrines of several mystics from Sheikh Al-Hujwiri (Lahore), Sheikh Bahauddin Zakariya (Multan), Baba Farid (Pakpattan), Bu Ali Qalandar (Panipat), Sheikh Ahmed Sirhindi (Sirhind) and was connected with networks of pilgrimage to the shrine of Muinuddin Chishti (Ajmer) and Jalaluddin Surkh Bukhari (Uch) towards the south and Nizamuddin Auliya (Delhi) and Sabir Pak (Kaliyar) to the east of Punjab. The region was also dotted with several popular shrines ascribed to local Pirs which also became increasingly popular. Thus colonial ethnographers could not ignore recording the dominant influence of popular shrines ascribed to Lalanwala Pir in the riverine plains of rural Punjab and Khwaja Khizr in the urban centres along the river Indus and its tributaries. The mystic landscape of Punjab produced ‘counter- hegemonic’ poetry of Bulleh Shah and Shah Husain and several others, on the one hand and qisse versifying legends of Hir-Ranjha, Sohni Mahiwal, etc on the other. -

The Islamia University of Bahawalpur, P a K I S T a N
IUB Scores 100% in HEC Online Classes Dashboard The Islamia University of Bahawalpur, P a k i s t a n Vol. 21 October–December, 2020 Khawaja Ghulam Farid (RA) Seminar and Mehfil-e-Kaafi | 17 Federal Minister for National Food Security Additional IG Police South Punjab Commissioner Bahawalpur Inaugurates Visits IUB Agriculture Farm | 04 Visits IUB | 04 4 New Buses | 09 MD Pakistan Bait ul Mal Visits IUB | 03 Inaugural Ceremony of the Project Punjab Information Technology Board Cut-Flower and Vegetable Production Praises IUB E-Rozgar Center | 07 Research and Training Cell | 13 Honourable Governor Advises IUB to be Student-Centric and Employee-Friendly Engr. Prof. Dr. Athar Mahboob, Vice Chancellor, briefed the Senate meeting of the University. particular, the Governor advised Vice Chancellor, the Islamia Honourable Chancellor about the The Governor appreciated the to University to be more student- University of Bahawalpur made a progress of the Islamia University performance of Islamia University centric and employee-friendly as courtesy call on Governor Punjab of Bahawalpur. Other matters of Bahawalpur and assured of his these ingredients were necessary and Chancellor of the University, discussed included the scheduling wholehearted support to Islamia for world-class universities. Chaudhary Muhammad Sarwar. of upcoming Convocation and University of Bahawalpur. In Governor Punjab Chaudhary Muhammad Sarwar exchanging views with Engr. Prof. Dr. Athar Mahboob, Vice Chancellor National Convention on Peaceful University Campuses Engr. Prof. Dr. Athar Mahboob, Vice Chancellor, the Islamia University of Bahawalpur attended the Vice Chancellor’s Convention on peaceful Universities held in joint collaboration of the Higher Education Commission of Pakistan and Inter University Consortium for Promotion of Social Sciences. -

CURRICULUM VITAE Dr. Muhammad Akram (Associate Professor)
CURRICULUM VITAE Dr. Muhammad Akram (Associate Professor) Chairperson, Department of Eastern Medicine, Directorate of Medical Sciences, Faculty of Life Sciences, Government College University Faisalabad-Pakistan Ex-Chairman, Department of Eastern Medicine, Faculty of Medical & Health Sciences, University of Poonch, Rawalakot Azad Kashmir, Pakistan. Coordinator, M. Phil and PhD in Eastern Medicine, Directorate of Medical and Health Sciences, Government College University Faisalabad, Pakistan CORRESPONDENCE DETAILS Contact Address: Department of Eastern Medicine, Directorate of Medical Sciences, Faculty of Science and Technology, Government College University Faisalabad. Permanent Address: Moza Nothain, Post office Chak Mubarak, Thana Bhera, Tehsil Bhalwal, District Sargodha Tel: Off: 0092-042-9200127: Res: (Mobile) 0092-0334-3367632; 0092-345-2888394; 0092- 0305-2049573 WhatsApp:+92-3452888394, IMO:+92-3452888394, Skype:hucon2008, Facebook:[email protected] Google scholar: [email protected] Researchgate: [email protected] Researcher ID:A-5679-2018 ORCID:0000-0001-7863-8803 E-mail: [email protected] ; [email protected] PERSONAL INFORMATION Name: Muhammad Akram Father Name: Muhammad Khan Date of Birth: February 12, 1980 Domicile: Sargodha, Punjab, Pakistan Religion: Islam National Identity Card No:42201-4270587-9 Qualification: B.E.M.S, M. Phil, Ph.D Area of Specialization: Eastern Medicine (Xanthine Oxidase Inhibition by Plants Extract and Clinical Efficacy of Herbal Formulation in Gouty Arthritis) Carrier Objective My objective is to be a part of an organization, which values merits, research skills, respects hard work and which can utilize my qualification and abilities for the mutual growth.And to remain embarked on the challenging field of eastern medicine where experience can be leveraged, knowledge and skills can be enhanced and humanity can be served in best possible way. -

Sufism in South Punjab, Pakistan: from Kingdom to Democracy
132 Journal of Peace, Development and Communication Volume 05, Issue 2, April-June 2021 pISSN: 2663-7898, eISSN: 2663-7901 Article DOI: https://doi.org/10.36968/JPDC-V05-I02-12 Homepage: https://pdfpk.net/pdf/ Email: [email protected] Article: Sufism in South Punjab, Pakistan: From kingdom to democracy Dr. Muzammil Saeed Assistant Professor, Department of Media and Communication, University of Author(s): Management and Technology, Lahore, Pakistan. Maria Naeem Lecturer, Department of Media and Communication, University of Management and Technology, Lahore, Pakistan. Published: 30th June 2021 Publisher Journal of Peace, Development and Communication (JPDC) Information: Saeed, M., & Naeem, M. (2021). Sufism in South Punjab, Pakistan: From kingdom to To Cite this democracy. Journal of Peace, Development and Communication, 05(02), 132–142. Article: https://doi.org/https://doi.org/10.36968/JPDC-V05-I02-12. Dr. Muzammil Saeed is serving as Assistant Professor at Department of Media and Communication, University of Management and Technology, Lahore, Pakistan. Corresponding Author’s Email: [email protected] Author(s) Note: Maria Naeem is serving as Lecturer at Department of Media and Communication, University of Management and Technology, Lahore, Pakistan. Email: [email protected] From kingdom to democracy 133 Abstract Sufism, the spiritual facet of Islam, emerged in the very early days of Islam as a self- awareness practice and to keep distance from kingship. However, this institution prospered in the times of Muslim rulers and Kings and provided a concrete foundation to seekers for spiritual knowledge and intellectual debate. Sufism in South Punjab also has an impressive history of religious, spiritual, social, and political achievements during Muslim dynasties. -

SED Verified Sites, Phase-VI, November,2014 Site No# District Tehsil UC Name UN No
SED Verified Sites, Phase-VI, November,2014 Site No# District Tehsil UC Name UN No. Chack/Moza Name Address 1 BWP Yazman Derawer Chack No. 118/DNB Chack No. 118/DNB, Yazman, BWP 2 BWP BWP 4/BC Basti Fazil Wali, Chack 4/BC Basti Fazil Wali, Chack No. 4/BC, P.O Dera Bakkha, BWP 3 Lodhran Lodhran Khanwan Basti Tibbi Ghalwan Basti Tibbi Ghullwan, P.O Qureshiwali, Tehsil/Distric Lodhran 4 Multan Jalalpur Lalwan Chak No.64/M Chak No.64/M 5 Okara Okara UC, Chak No 37/4A.L 41 Chak No. 39/4A.L Chak No. 39/4A.L, Gamabar,Okara 6 Okara Okara UC, Chak No 40/4A.L 42 Chak No. 41/4A.L Chak No. 41/4A.L, Gamabar,Okara Basti HABIB UR Rehman chandia 7 R.Y.Khan Khan pur Din pur sharif Basti HABIB UR Rehman chandia markaz zahir pir markaz zahir pir 8 BWN Chishtian Kalia Shah Ada Mari Shock Shah Ada Mari Shock Shah, BWN Road, Chishtian Basti Bashi, Moza Shahbaz, Boys Degree College Road, Near 9 BWN Minchinabad Minchinabad II 2 Basti Bashir Petrol Pump, Minchinabad 10 BWN Minchinabad Said Ali 114 Ada Feeder Ada Feeder, BWN Road, Minchinabad 11 BWN Minchinabad Meclod Gunj 106 Ahmad Pur Ahmad pur meclod gunj Minchinabad 12 BWN Minchinabad Mirzika 111 Tara Cheena Tara cheena mirzika minchinabad 13 BWN Minchinabad Bunga akhtar Nehal 112 Mahraj khurd Mehraj khurd mari akhtar nehaal 14 Gujrat Kharian Warichanwala 93 Soli wind Soli wind , Via Mangowal Road Dinga, Tehsil Kharian 15 Chiniot Chiniot 153 /JB 19 143 Chak 143 Chak No Jhoke Kalra Chiniot 16 Chiniot Chiniot Harsa Sheikh 11 Masoor Ke Masoor Ke Near Mal Ke Asiyan Chiniot Chak Gangi pur, Adda Rang Shah, Pakpattan Road, Arifwala, 17 Pakpattan Arifwala Chak No 13/EB 44 Chak Gangi pur Pakpattan 18 Khanewal Khanewal 74/15-L / No. -

Muslim Mystics and Sufi Silsilahs in the India
MUSLIM MYSTICS AND SUFI SILSILAHS IN THE INDIA. By Dr.Amit Dey. When I am writing this article during the beginning of the new year, 2015, the world and the Indian subcontinent are going through certain uneasy experiences ranging from the violent reaction in the aftermath of the publication of cartoons in some western newspapers allegedly hurting the sentiment of millions of Muslims all over the world, when quite a few innocent school children perished in a brutal attack in Peshawar, when the Indian state of West Bengal is trying to recover from the infamous Khagragarh (in Burdwan district) explosion, when certain south asian regions are being exposed for the alarming imbalance in sex ratio confirming the prevalence of female foeticide even during the second decade of the twenty first century, the stereotyping of Islam as an intolerant, homogeneous and violent religion gathered momentum in the oriental and occidental worlds.1 In the context of these developments at the regional and trans-regional levels, the study of Islamic mysticism is becoming more and more relevant particularly due to the tolerant, spiritual and humane outlook of the Muslim mystics and the appropriating and accommodating nature of various sufi orders which created ripples in the socio-spiritual world of the subcontinent. Ironically, the sufis are virtually eliminated from the world of their origin, i.e., Central Asia and the Middle East. However, in South Asia where the Muslim mystics once took spiritual refuge, Sufism is still vibrant as a spiritual and cultural force. Existence of Sufism proves that like any other religion, Islam is also characterized by heterogeneity. -
To View NSP QAT Schedule
EMIS CODE New QAT Program Sr. No Shift Time SCHOOL NAME Address TEHSIL DISTRICT REGION QAT Day /SCHOOL CODE Date Name 12.30 pm NEW AGE PUBLIC UC Name Dhurnal, UC # 39, Moza FATEH 1 B ATK-FJG-NSP-VIII-3061 ATTOCK North 14 18.12.18 NSP to 2.30 pm SCHOOL Dhurnal, Chak / Basti Dhurnal Ada, JANG Tehsil Fateh Jung District Attock 12.30 pm Village Bai, PO Munnoo Nagar, Tehsil HASSANAB 2 B ATK-HDL-NSP-IV-210 Sun Rise High School ATTOCK North 11 14.12.18 NSP to 2.30 pm Hassan Abdal, District Attock DAL 12.30 pm Science Secondary Thatti Sado Shah, Po Akhlas, Tehsil PINDI 3 B ATK-PGB-NSP-IV-214 ATTOCK North 16 20.12.18 NSP to 2.30 pm School Pindi Gheb, District Attock GHEB 12.30 pm Al Aziz Educational Village Gangawali, Teshil Pindi Gheb, PINDI 4 B ATK-PGB-NSP-IV-216 ATTOCK North 17 09.01.19 NSP to 2.30 pm School System District Attock GHEB Basti Haider town(Pindi Gheb), Mouza 12.30 pm PINDI 5 B ATK-PGB-NSP-VII-2477 Hamza Public School Pindi Gheb, UC Name Chakki, UC # 53, ATTOCK North 17 09.01.19 NSP to 2.30 pm GHEB Tehsil Pindi Gheb, District Attock. Mohallah Jibby. Village Qiblabandi, PO 12.30 pm Tameer-e-Seerat Public 6 B ATK-HZO-NSP-IV-211 Kotkay, Via BaraZai, Tehsil Hazro, HAZRO ATTOCK North 12 15.12.18 NSP to 2.30 pm School District Attock 9.00 am to Stars Public Elementary 7 A ATK-ATK-NSP-IV-207 Dhoke Jawanda, Tehsil & District Attock ATTOCK ATTOCK North 12 15.12.18 NSP 11.00 School 12.30 pm Muslim Scholar Public Dhoke Qureshian, P/O Rangwad, tehsil 8 B ATK-JND-NSP-VI-656 JAND ATTOCK North 15 19.12.18 NSP to 2.30 pm School Jand 12.30 pm Farooqabad -

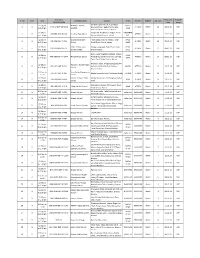
DERA-GHAZI-KHAN-Rend60.Pdf
Renewal List S/NO REN# / NAME FATHER'S PRESENT ADDRESS DATE OF ACADEMIC REN DATE NAME BIRTH QUALIFICATION 1 39939 ZAFAR PEER BUKHSH CHAK TOPI WALA MOUZA KHAKHI P/O SAMEDISTT. 7-10-1984 MATRIC 13/7/2014 HUSSAIN D.G. KHAN , DERA GHAZI KHAN, PUNJAB 2 37693 ABDUL RASHID ELAHI BAKHSH IDREES TOWN GADAI SHUMALI P/O SAMIN TEH & 1-10-1970 MATRIC 14/7/2014 DISTT. D.G. KHAN , DERA GHAZI KHAN, PUNJAB 3 21707 ABID HUSSAIN KHADIM CHOTI ZARIN BASTI MARI WALAP.O. HASSAN ABAD, 10/5/1978 MATRIC 14/07/2014 HUSSAIN DERA GHAZI KHAN, PUNJAB 4 32701 SAAD ULLAH MUHAMMAD H/NO. 92 BLOCK NO. 15 EID GAH ROAD D, G, KHAN , 26-12- MATRIC 15/07/2014 SAFDAR SAFDAR DERA GHAZI KHAN, PUNJAB 1975 5 47704 GHULAM ALLAH WASAYA VILL, BAQIR WALA P/O NUTKANI TEH, TAUNSA 7-7-1978 MATRIC 19/07/2014 MUSTAFA SHARIF DISTT,, DERA GHAZI KHAN, PUNJAB 6 31539 MUNIR AHMED MOLVI BASHIR MORAH P/O NANKANI TEH, TOUNSAH DISTT, D G 28-5-1973 MATRIC 04/09/2014 AHMED KHAN, DERA GHAZI KHAN, PUNJAB 7 22237 SALEEM MIRZA H.N.82BLOCK G, DERA GHAZI KHAN, PUNJAB 1/4/1974 MATRIC 20/09/2014 AKHTAR MUHAMMAD RAFIQ 8 37680 MUHAMMAD MUHAMMAD HOUSE NO. 92, BLOCK -9, , DERA GHAZI KHAN, 31-8-1968 MATRIC 23/9/2014 SALEEM SHARIF PUNJAB SHARIF 9 40134 MUHAMMAD MUHAMMAD H NO. 102 BLOCK F D G KHAN, DERA GHAZI KHAN, 24-2-1984 MATRIC 27/9/2014 ALI ANSARI RIAZ ANSARI PUNJAB 10 40135 MUHAMMAD MUHAMMAD BLOCK NO. -

The Mystic Path of Shaikh Bahauddin Zakariya
International Journal Online of Humanities (IJOHMN) ISSN: 2395-5155 Volume 5, Issue 4, August 2019 DOI: https://doi.org/10.24113/ijohmn.v5i5.115 The Mystic Path of Shaikh Bahauddin Zakariya Dr. R. Subramony Associate Professor and Head of the Department Department of English (Autonomous) Madurai, Tamil Nadu, India [email protected] Abstract Shaikh Bahauddin Zakariya (1182-1262) laid the foundation of the Suhrawardi order in Multan, which played a significant role in the socio cultural history of north-western India. His ancestors had migrated from Mecca and settled in Multan. His father Shaikh Wajihuddin was married to the daughter of Maulana Husamuddin Tirmizi, who had migrated to Punjab in the wake of the Mongol invasions. Bahauddin Zakariya was born at Kot Karor, a village near Multan. While still a young boy, he memorized the Quran and learnt to recite it in seven styles of recitation. During a long stay in the famous centres of education – Khurasan, Bukhara, Madina and Palestine- he studied the traditional subjects. Keywords- Shaikh Bahauddin Zakariya, Suhrawardis, Shaikh Nizamuddin Auliya Humaira Arif Dasti states, “Shaikh Bahauddin Zakariya (1182-1262) laid the foundation of the Suhrawardi order in Multan, which played a significant role in the sociocultural history of www.ijohmn.com 90 International Journal Online of Humanities (IJOHMN) ISSN: 2395-5155 Volume 5, Issue 4, August 2019 north-western India. His ancestors had migrated from Mecca and settled in Multan. His father Shaikh Wajihuddin was married to the daughter of Maulana Husamuddin Tirmizi, who had migrated to Punjab in the wake of the Mongol invasions.