Commission Implementing Regulation (EU)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
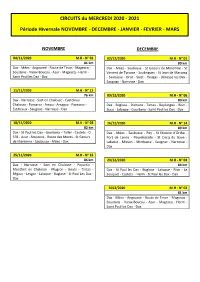
CIRCUITS Du MERCREDI 2020 - 2021
CIRCUITS du MERCREDI 2020 - 2021 Période Hivernale NOVEMBRE - DECEMBRE - JANVIER - FEVRIER - MARS NOVEMBRE DECEMBRE 04/11/2020 M.H - N° 02 02/12/2020 M.H - N° 01 81 km 80 km Dax - Mées - Angoumé - Route de Tinon - Magescq - Dax - Mées - Saubusse - St Geours de Maremne - St Soustons - Vieux-Boucau - Azur - Magescq - Herm - Vincent de Tyrosse - Saubrigues - St Jean de Marsacq Saint Paul les Dax - Dax - Saubusse - Orist - Siest - Heugas - Bénesse les Dax - Saugnac - Narrosse - Dax 11/11/2020 M.H - N° 13 76 km 09/12/2020 M.H - N° 06 Dax - Narrosse - Sort en Chalosse - Castelnau 80 km Chalosse - Pomarez - Amou - Arsague - Pomarez - Dax - Buglose - Pontonx - Tartas - Beylongue - Rion - Estibeaux - Saugnac - Narrosse - Dax Boos - Laluque - Gourbera - Saint Paul les Dax - Dax 18/11/2020 M.H - N° 03 16/12/2020 M.H - N° 14 82 km 80 km Dax - St Paul les Dax - Gourbera – Taller - Castets - D Dax - Mées - Saubusse - Pey - St Etienne d'Orthe - 378 - Azur - Soustons - Route des Monts - St Geours Port de Lanne - Peyrehorade - St Cricq du Gave - de Maremne - Saubusse - Mées - Dax Labatut - Misson - Mimbaste - Saugnac - Narrosse - Dax 25/11/2020 M.H - N° 15 86 km 23/12/2020 M.H - N° 04 Dax - Narrosse - Sort en Chalosse - Poyartin - 84 km Montfort en Chalosse - Mugron - Gouts - Tartas - Dax - St Paul les Dax - Buglose - Laluque - Rion - Le Bégaar - Lesgor - Laluque - Buglose - St Paul Les Dax - Souquet - Castets - Herm - St Paul les Dax - Dax Dax 3012/2020 M.H - N° 02 81 km Dax - Mées - Angoumé - Route de Tinon - Magescq - Soustons - Vieux-Boucau - Azur -

PLUI) Sud Du Territoire : Avis Sur Le Projet Après Arrêt
COMMUNE DE CAUBIOS-LOOS DCM 2019/ 03 - 64 230 - DÉLIBÉRATIONS DU CONSEIL MUNICIPAL L’an deux mille dix-neuf, le 6 mai à 20 heures 30, les membres du Conseil Municipal de la Commune de CAUBIOS-LOOS se sont réunis, en salle de la mairie, sous la Présidence de Monsieur Bernard LAYRE, Maire. Étaient présents : Mmes DESCHASEAUX Brigitte, CONSTANS Grace, MONARET Marie-Hélène, ARNAUDET (PHILIPPERON) Virginie, BELTRAN Sabine, MM VANDERBEEKEN Francis, BRUNET Gilles, JOUBERT Patrick, LESQUIBE Sébastien, Absents excusés : M. LAHITTE Olivier, MAUMELLE Julien, PÉRÉ Fabien , Mmes LACOSTE Jeanine, CASTAING Yvette Convocation du 30/04/2019. DCM 2019 / 03 / 01 – Plan Local d’Urbanisme Intercommunal (PLUI) Sud du Territoire : Avis sur le projet après arrêt M. le Maire rappelle que le PLUi a été prescrit par délibération du Conseil communautaire en date du 10 décembre 2015. Les objectifs poursuivis, les modalités de concertation avec la population ainsi que les modalités de collaboration avec les communes membres ont été à cette occasion définies. Au moment de la prescription, la Communauté de communes des Luys en Béarn était alors composée de 22 communes soit Argelos, Astis, Aubin, Auga, Auriac, Bournos, Carrère, Claracq, Doumy, Garlède-Mondebat, Lalonquette, Lasclaveries, Lème, Miossens-Lanusse, Montardon, Navailles-Angos, Sauvagnon, Serres-Castet, Sévignacq, Pouliacq, Thèze et Viven. Le territoire communautaire a ensuite évolué par : *une adhésion des communes de Caubios-Loos et de Momas à la Communauté de communes des Luys en Béarn le 29 décembre 2016. *une fusion avec les Communautés de communes du Canton d’Arzacq et du Canton de Garlin le 1er janvier 2017. A l’issue de cette évolution, le choix d’élaborer un PLUi sur le périmètre de la nouvelle Communauté de communes composée désormais de 66 communes n’a pas été retenu. -

247 Communes Soumise À Zones IAHP 23/04/2021 14:00 ABIDOS
247 communes soumise à zones IAHP 23/04/2021 14:00 Situation Date indicative ZP Commune N° Insee Type de zone stabilisée ou remise en place coalescente évolutive palmipèdes ABIDOS 64003 ZS Stabilisée ABITAIN 64004 ZS Stabilisée 14/05/2021 ABOS 64005 ZS Stabilisée ANDREIN 64022 ZS Stabilisée 14/05/2021 ANOS 64027 ZSR Stabilisée 13/05/2021 ARANCOU 64031 ZS Évolutive ARAUJUZON 64032 ZS Stabilisée 14/05/2021 ARAUX 64033 ZS Stabilisée 14/05/2021 ARBERATS-SILLEGUE 64034 ZS Stabilisée 14/05/2021 ARBOUET-SUSSAUTE 64036 ZS Stabilisée 14/05/2021 ARGAGNON 64042 ZS Stabilisée ARGELOS 64043 ZS Stabilisée ARGET 64044 ZSR Stabilisée 13/05/2021 ARNOS 64048 ZSR Stabilisée 13/05/2021 ARRAUTE-CHARRITTE 64051 ZS Évolutive ARRICAU-BORDES 64052 ZS Stabilisée 11/05/2021 ARROS-DE-NAY 64054 ZS Stabilisée 17/05/2021 ARROSES 64056 ZS Stabilisée 11/05/2021 ARTHEZ-D'ASSON 64058 ZS Stabilisée 17/05/2021 ARTHEZ-DE-BEARN 64057 ZSR Stabilisée 13/05/2021 ARTIX 64061 ZS Stabilisée ARUDY 64062 ZS Stabilisée 17/05/2021 ARZACQ-ARRAZIGUET 64063 ZSR Stabilisée 13/05/2021 ASSON 64068 ZS Stabilisée 17/05/2021 ASTE-BEON 64069 ZS Stabilisée 17/05/2021 ASTIS 64070 ZS Stabilisée ATHOS-ASPIS 64071 ZS Stabilisée 14/05/2021 AUBIN 64073 ZSR Stabilisée 13/05/2021 AUBOUS 64074 ZS Stabilisée 11/05/2021 AUDAUX 64075 ZS Stabilisée 14/05/2021 AUGA 64077 ZSR Stabilisée 13/05/2021 AURIAC 64078 ZS Stabilisée AURIONS-IDERNES 64079 ZS Stabilisée 11/05/2021 AUTERRIVE 64082 ZS Évolutive AUTEVIELLE-ST-MARTIN-BIDEREN 64083 ZS Stabilisée 14/05/2021 AYDIE 64084 ZS Stabilisée 11/05/2021 AYDIUS 64085 ZS -

COMMUNAUTÉ DE COMMUNES ADOUR MADIRAN Procès-Verbal
Département des Hautes-Pyrénées COMMUNAUTÉ DE COMMUNES ADOUR MADIRAN Procès-verbal de séance Conseil Communautaire du 02 février 2017 Membres en exercice : 99 Date de la convocation : 25/01/2017 Sous la présidence de Monsieur Frédéric RÉ, Président Présents : Aline ABADIE, Vincent ABADIE, Roland ARTUS, Marie BAUDOIN, Patrick BAYLERE, Frédérique BELLARDI-SAVOYE, Martine BETBEZE, Jacques BETTONI, Franck BOCHER, Sylvie BOIRIE, Annie BONNECARRERE, Alain BONNECARRERE, Maryse BORDIER, Monique BOSOM, Christian BOURBON, Bruno CAMPAGNARI, Sidonie CARDOUAT, Alain CASSOU, Jean CAUBIOS, Serge COURNET, Jean-Pierre CURDI, Jean-Louis CURRET, Didier CUVILIER, José DEBAT, Gérard DIEUZEIDE, Louis DINTRANS, Christian DUBERTRAND, Sylvie DUBERTRAND, Roland DUBERTRAND, Sandra DUCES, Gilbert DUCOS, Jacques DUFFAU, Christian DHUGUES, Guy DULOUT, Olivier EUDES, Marc FRATTA, Denis GRONNIER, Catherine GUILLON-MARIENVAL, Alain GUILLOUET, Christine HABAS, Eric JOSEPH, Serge JOSEPH, Joël LACABANNE, Julien LACAZE, Jean-Marc LAFFITTE, Claude LAFFONTA, Dominique LAGAHE, Paul LAGRAVE, Antoine LAPEZE, Bernard LAQUAY, Anne-Laure LARMITOU-LATRILLE, Francis LARRANG, Bernard LAURENS, Francis LELAURIN, Francis LOUMAGNE, Bernard LUSSAN, Alain MADRONA, Jean-Louis MAGNI, Pierre MANHES, Jérôme MARRE, Yves MENJOULOU, Michel MENONI, Jean NADAL, Laurent NICOLAU, René NOGUERE, Denise NOGUES-CHARTRAIN, Pascal PAUL, Francis PEDAUGE, Jean-Paul PENE, Thérèse PEYCERE, Francis PLÉNACOSTE, Magali POINSOT-DARGAIGNON, Bernard POUBLAN, Christian PUYO, Frédéric RÉ, Pierre RENON, Charles ROCHETEAU, -

Mirande-Astarac
Gers Solidaire Mirande-Astarac Informations Supercie : 467 km2 Population : 12705 habitants Conseillers départementaux : Communes du canton AUX-AUSSAT (http://www.gers.fr/information- transversale/annuaire-des-communes/aux-aussat- 11564) LAMAZERE (http://www.gers.fr/information- transversale/annuaire-des-communes/lamazere- 11581) LOUBERSAN (http://www.gers.fr/information- transversale/annuaire-des-communes/loubersan- 11582) MALABAT (http://www.gers.fr/information- transversale/annuaire-des-communes/malabat-11583) MANAS-BASTANOUS (http://www.gers.fr/information- transversale/annuaire-des-communes/manas- bastanous-11584) MARSEILLAN (http://www.gers.fr/information- transversale/annuaire-des-communes/marseillan- 11585) MIELAN (http://www.gers.fr/information- transversale/annuaire-des-communes/mielan-11586) MIRAMONT-D'ASTARAC (http://www.gers.fr/information- transversale/annuaire-des-communes/miramont- dastarac-11587) MIRANDE (http://www.gers.fr/information- transversale/annuaire-des-communes/mirande- 11588) MONCASSIN (http://www.gers.fr/information- transversale/annuaire-des-communes/moncassin- 11589) MONT-DE-MARRAST (http://www.gers.fr/information- transversale/annuaire-des-communes/mont-de- marrast-11591) MONTEGUT-ARROS (http://www.gers.fr/information- transversale/annuaire-des-communes/montegut- arros-11592) . https://www.gerssolidaire.org/informations-transversales/annuaire-des-cantons/mirande-astarac-11229? arros-11592) PONSAMPERE (http://www.gers.fr/information- transversale/annuaire-des-communes/ponsampere- 11593) -

Des Paroisses De Sainte-Fauste-Du Fezensac Et De Saint-Pierre-De-Barran-Jégun
magazine■des■paroisses■de■Sainte-Fauste-du■Fezensac■et■de■Saint-pierre-de-barran-jégun 97 ■ n° carillon ■de nos vallées e ■ 2,50 ■ - ■ 2015 ■ septembre-octobre ■ - ■ L’église■est■ouverte imestriel à■tous■les■âges b ■■ACTUALITéS ■■SAInT-pIerre-de- ■■CéLébrATIonS Lourdes 2015 bArrAn-jégUn Horaires de Toussaint La joie de la mission Un jeune domaine viticole et prières aux cimetières page 3 page 10 page 12 jUbILé■de■LA■mISérICorde ParAboLeS■32 des■semences■de■réconciliation■ pages■II■-■III 2 Actualités édito Catéchisme pour adulte entreprenons■! des■rencontres■riches■ Que l’on soit jeune ou dans l’âge mûr, il n’y a pas d’âge pour s’initier à la vie chrétienne ! de■découvertes Et des adultes n’hésitent pas à continuer leur initiation, Ils sont quatre adultes – Nelly, Claire, Benoît et Aurore – en se préparant à la communion et la confirmation. et se préparent à la confirmation. Aurore revient sur ces temps L’été des rencontres familiales de rencontre où bonne humeur et spiritualité sont toujours et amicales est maintenant rangé au rendez-vous. dans les cartons de souvenirs et les cartes mémoires des appareils photos. Mais ce temps d’automne nous pousse à entreprendre la récolte des fruits, des raisins et des amitiés renouvelées. Aussi à se laisser gagner par les propositions d’engagement dans l’Église paroissiale ou plus largement dans le service des plus démunis, et il n’en manque Des lycéens au service de la catéchèse. pas ! Les services rendus aux hommes et aux femmes de notre monde sont le plus bel exemple ette année dans nos deux paroisses, savoir plus sur la vie de Jésus, de Dieu et s’est formé un groupe de catéchu- sur les chrétiens. -

40990 Angoumé 40150 Angresse 40320 Arboucave 40110
40990 Angoumé 40150 Angresse 40320 Arboucave 40110 Arengosse 40700 Argelos 40430 Argelouse 40110 Arjuzanx 40330 Arsague 40190 Arthez-d'Armagnac 40120 Arue 40700 Aubagnan 40500 Audignon 40400 Audon 40320 Bahus-Soubiran 40380 Baigts 40700 Bassercles 40360 Bastennes 40320 Bats 40400 Bégaar 40410 Belhade 40120 Bélis 40300 Bélus 40180 Bénesse-lès-Dax 40230 Bénesse-Maremne 40250 Bergouey 40240 Betbezer-d'Armagnac 40370 Beylongue 40700 Beyries 40390 Biarrotte 40170 Bias 40390 Biaudos 40600 Biscarrosse 40330 Bonnegarde 40370 Boos 40270 Bordères-et-Lamensans 40190 Bourdalat 40330 Brassempouy 40320 Buanes 40120 Cachen 40300 Cagnotte 40430 Callen 40090 Campagne 40090 Canenx-et-Réaut 40400 Carcarès-Sainte-Croix 40400 Carcen-Ponson 40380 Cassen 40330 Castel-Sarrazin 40360 Castelnau-Chalosse 40320 Castelnau-Tursan 40700 Castelner 40500 Cauna 40300 Cauneille 40250 Caupenne 40700 Cazalis 40090 Cère 40320 Classun 40320 Clèdes 40180 Clermont 40210 Commensacq 40240 Créon-d'Armagnac 40360 Donzacq 40500 Dumes 40210 Escource 40290 Estibeaux 40240 Estigarde 40320 Eugénie-les-Bains 40500 Eyres-Moncube 40500 Fargues 40190 Frêche 40350 Gaas 40090 Gaillères 40380 Gamarde-les-Bains 40420 Garein 40180 Garrey 40110 Garrosse 40160 Gastes 40330 Gaujacq 40320 Geaune 40090 Geloux 40380 Gibret 40180 Goos 40990 Gourbera 40465 Gousse 40400 Gouts 40290 Habas 40700 Hagetmau 40300 Hastingues 40250 Hauriet 40180 Heugas 40190 Hontanx 40700 Horsarrieu 40230 Josse 40700 Labastide-Chalosse 40300 Labatut 40530 Labenne 40210 Labouheyre 40320 Lacajunte 40700 Lacrabe 40090 Laglorieuse -

Légende Nestalas Uz Soulom Arcizans- Limites Cantonales Avant Villelongue Beaucens Limites Communales
N PLAN LOCAL D’URBANISME Ouzous Salles Agos- COMMUNE DE CAUTERETS Vidalos Ayzac- LOCALISATION Sère-en-Lavedan Ost Boô- Gez Silhen Saint- Pastous ARGELES-Ayros- GAZOST Arbouix Lau-Préchac Balagnas Vier- Saint- Bordes Savin Adast Artalens- Souin Pierrefitte- Légende Nestalas Uz Soulom Arcizans- Limites cantonales Avant Villelongue Beaucens Limites communales Surface communale Cauterets Saint- Lanne Castelnau- Rivière-Basse Madiran Hères Soublecause Labatut- Rivière Hagedet Caussade- Rivière Villefranque Lascazères Estirac Auriébat Sombrun Sauveterre Maubourguet Lahitte- Toupière Vidouze Monfaucon Larreule Lafitole Buzon Gensac Nouilhan Ansost Barbachen Caixon Liac Artagnan Ségalas Vic-en- Villenave- Bigorre Sarriac- Rabastens- près- Sanous Bigorre de-Bigorre Béarn Saint- Mingot Lézer Bazillac Estampures Escaunets Camalès Fréchède Lacassagne (Canton de Vic-en-Bigorre) Sénac Moumoulous Escondeaux Villenave- Saint-Sever- Mazerolles Talazac Pujo près- Ugnouas Marsac de-Rustan Séron Tarasteix Lescurry Mansan Bernadets- Marsac Tostat Bouilh- Debat Siarrouy Peyrun Sarniguet Castéra-Lou Laméac Devant Fontrailles Andrest Antin Lagarde Soréac Trouley- Oroix Gayan Jacque Aurensan Dours Bouilh- Labarthe Lapeyre Trie-sur- Louit Péreuilh Lubret- Gardères Pintac Chis Saint-Luc Lalanne- Baïse Sadournin Guizerix Peyret- Oursbelille Bazet Osmets Trie Collongues Marseillan Saint-André (Canton d'Ossun) Sabalos Luby- Sariac- Orleix Chelle- Betmont Vidou Larroque Casterets Bours Debat Puntous Magnoac Luquet Bordères- Oléac-Debat Castelvieilh Mun Thermes- sur-l'Echez -

Gîte Four À Pain - Louer - Chalosse - Landes
GÎTE FOUR À PAIN - LOUER - CHALOSSE - LANDES GÎTE "FOUR À PAIN" À LOUER - LANDES CHALOSSE 5 personnes http://gite-fourapain-chalosse.fr Michel ROQUELAURE +33 6 01 85 97 77 A Maison "Four à pain" à Louer - Landes Chalosse : 85 bis route de Lavielle 40380 LOUER Maison "Four à pain" à Louer - Landes Chalosse Maison 5 2 70 personnes chambres m2 (Maxi: 5 pers.) Vous voulez passer votre séjour en Landes Chalosse dans un endroit particulier ? Bonne pioche ! A Louer, Michel a rénové cette maison en conservant le four à pain familial. Cette location meublée vous offre calme et confort. Vous trouverez une grande pièce à vivre, où l'on voit le four à pain, la cuisine est indépendante et correspond à l'entrée principale. L'établissement thermal de Préchacq- les-Bains est à 3,7 km, vous pourrez y aller en vélo (local fermé à l'extérieur de la location). Les commerces et services se trouvent à 10 minutes en direction de Pontonx-sur-Adour ou 15 minutes à Montfort-en-Chalosse. A Louer, vous profiterez des lieux de pêche et des circuits de randonnées pédestres et VTT (rando guide n°4 en vente à l'office de tourisme). Le petit plus : la présence d'un ancien four à pain dans le séjour et la possibilité de l'utiliser avec l'accord du propriétaire. Pièces et équipements Chambres Chambre(s): 2 Lit(s): 2 dont lit(s) 1 pers.: 0 dont lit(s) 2 pers.: 2 1 convertible 2 personnes Salle de bains / Salle Salle de bains avec douche Sèche cheveux Infos sur l'établissement d'eau Salle(s) de bains (avec baignoire): 1 A proximité propriétaire Entrée indépendante WC -

Gascogne, Carte Des Communautés
CARTE DES PAYS ET COMMUNAUTES DE COMMUNES DU GERS PERGAIN-TAILLAC LIGARDES POUY-ROQUELAURE SAINT-ANTOINE SAINT-MEZARD GIMBREDE SEMPESSERRE FLAMARENS GAZAUPOUY BERRAC MIRADOUX SAINT-MARTIN-DE-GOYNE FOURCES SAINTE-MERE MIRADOUX PEYRECAVE LA ROMIEU LARROQUE-ENGALIN CASTERA-LECTOUROIS SAINT-AVIT-FRANDAT LABARRERE LARROQUE-SUR-L'OSSE CASTET-ARROUY CONDOM LAGARDE CONDOM CASTELNAU-SUR-L'AUVIGNON CAZAUBON MONTREAL LECTOURE LA TENAREZE CASTELNAU-D'AUZAN CAUSSENS PLIEUX MARSOLAN MONTREAL BEAUMONTLARRESSINGLE LECTOURE LAURAET MONCLAR BERAUT BLAZIERT L'ISLE-BOUZON MAULEON-D'ARMAGNAC LAREE LANNEMAIGNAN SAINT-ORENS-POUY-PETIT SAINT-CREAC ROQUEPINE CASSAIGNE MOUCHAN TERRAUBE MAUROUX GRAND ARMAGNAC CASTERON BRETAGNE-D'ARMAGNACCAZENEUVE LAGRAULET-DU-GERS SAINT-CLAR MARGUESTAU MAIGNAUT-TAUZIA MAS-D'AUVIGNON MAGNAS CAZAUBON GONDRIN ESTANG PAUILHAC CASTEX-D'ARMAGNAC REANS Sans regroupement MANSENCOME CASTELNAU-D'ARBIEU COEUR DE LOMAGNE LIAS-D'ARMAGNAC AVEZAN GAUDONVILLE CAMPAGNE-D'ARMAGNAC VALENCE-SUR-BAISE MONGUILHEM AYZIEU EAUZE SAINT-PUY LAMOTHE-GOAS SAINT-CLAR Pays d'Armagnac URDENS TOURNECOUPE MAUPAS SAINT-LEONARD PESSOULENS COURRENSAN LA SAUVETAT ROQUES LAGARDERE FLEURANCE PANJAS BEAUCAIRE SAINTE-RADEGONDE BOURROUILLAN FLEURANCE BRUGNENS TOUJOUSEMONLEZUN-D'ARMAGNAC VALENCE-SUR-BAÎSEAYGUETINTE EAUZE LARROQUE-SAINT-SERNIN ESTRAMIAC CADEILHAN MANCIET BIVES AVENSAC SALLES-D'ARMAGNAC BEZOLLES RAMOUZENS JUSTIAN LA LOMAGNE GERSOISE LAUJUZAN LANNEPAX REJAUMONT NOULENS CERAN MORMES BAJONNETTE HOMPS SAINTE-CHRISTIE-D'ARMAGNAC ROZES CEZAN GOUTZ BASCOUS MOUREDE -

Corrigé THÈME 1 LES Transformations Du Paysage
livret du jeune visiteur élèves du cycle 3 corrigé THÈME 1 LES transformations du paysage 1 COMMENSACQ La Mouleyre. Bergers près d’un parc Sans date, réf M.A. : 66.27.2186. Juillet 2005. 1 - Complète le tableau ci-dessous en faisant la liste des éléments vus sur les photographies 2 - Réponds aux questions suivantes : - A quoi servait la construction de la photographie prise par Félix Arnaudin ? Photographie en noir et blanc Photographie en couleur A parquer les moutons. La bergerie La maison Les moutons La moto - A quoi servait la construction de la photographie prise par Jean-Joël Le Fur ? A se loger. Les bergers sur échasses L’homme assis à l’ombre - A ton avis, quel était l’intérêt au XIXe siècle de se déplacer dans la lande avec les échasses comme les bergers ? La forêt au lointain Les chênes autour de la maison A faire de grands pas, voir au loin, enjamber les cours d’eau et les fossés. La forêt de pins au lointain 3 - Entoure sur les deux photos les moyens que les hommes utilisent pour se déplacer. Les échasses, la moto. THÈME 1 2 MORCENX Pêche au Lagouat à Cornalis 1 – Entoure sur les trois photographies la construction qui a servi de point de repère à Jean-Joël Le Fur. La bergerie. 2 – Réponds à la question suivante : Quel élément nouveau observe-t-on sur la photographie couleur (B) ? La forêt. A 13 février 1898, réf M.A. : 66.27.2163. 3 – Coche les bonnes réponses. Photographie Photographie en noir et blanc (A) en couleur (B) Lande rase ✓ Espace vide ✓ Espace plein ✓ Noir et blanc ✓ Couleur ✓ Forêt ✓ Eau ✓ 4 – Réponds aux questions suivantes : Quel événement est survenu avant la prise de la photographie appelée reconduite (C) ? La tempête KLAUS. -

Liste Des Services D'aide À Domicile
14/04/2015 LISTE DES SERVICES D'AIDE À DOMICILE pouvant intervenir auprès des personnes âgées bénéficiaires de l'Allocation Personnalisée d'Autonomie (A.P.A.), des adultes handicapées bénéficiaires de la Prestation de Compensation du Handicap (P.C.H.) et pour certains, auprès des bénéficiaires de l'aide ménagère au titre de l'aide sociale légale départementale A Habilitation à l'aide sociale départementale www.cg64.fr TI = Type d'interventions réalisables u AS = Service prestataire d'aide à domicile pouvant intervenir auprès des P = Prestataire -- M = Mandataire t bénéficiaires de l'aide sociale départementale Pour plus d'information, voir : Choisir un mode d'intervention . C Code AS Nom du service Adresse Ville Téléphone TI Territoire d'intervention G Postal CCAS Hôtel de ville P Ville d’ANGLET A AS 64600 ANGLET 05 59 58 35 23 Centre Communal d'Action Sociale Place Charles de Gaulle M Département des Pyrénées-Atlantiques Uniquement en garde de nuit itinérante Association 12, rue Jean Hausseguy A AS 64600 ANGLET 05 59 03 63 30 P Communauté d’agglomération du BAB (BIARRITZ, Les Lucioles BP 441 BAYONNE, ANGLET) et périphérie proche Association P 95, avenue de Biarritz 64600 ANGLET 05 59 41 22 98 Département des Pyrénées-Atlantiques Côte Basque Interservices (ACBI) M Association P 3, rue du pont de l'aveugle 64600 ANGLET 05 59 03 53 31 Département des Pyrénées-Atlantiques Services aux Particuliers (ASAP) M Association 12, rue Jean Hausseguy P 64600 ANGLET 05 59 03 63 30 Département des Pyrénées-Atlantiques Garde à Domicile BP 441 M Centre