US 2019 / 0216870 A1 Witowski Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

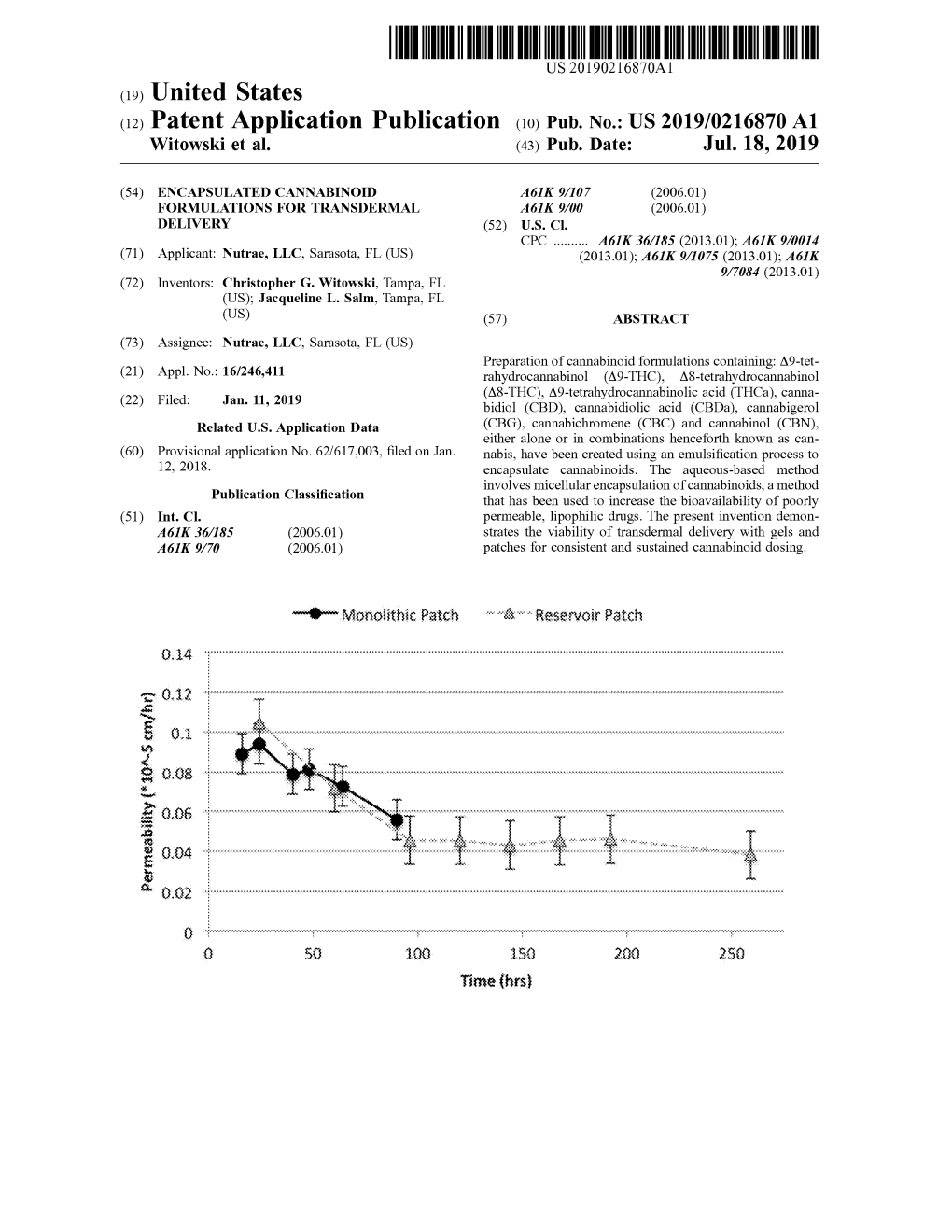
(12) United States Patent (10) Patent No.: US 9.435,817 B2 Benchikh Et Al
USOO9435817B2 (12) United States Patent (10) Patent No.: US 9.435,817 B2 Benchikh et al. (45) Date of Patent: Sep. 6, 2016 (54) DETECTION OF SYNTHETIC OTHER PUBLICATIONS CANNABINOIDS C. V. Rao, “Immunology. A textbook”. Alpha Science Internatl. Ltd., 2005, pp. 63, 69-71.* (75) Inventors: Elouard Benchikh, Crumlin (GB); Weissman et al., “Cannabimimetic activity from CP-47,497, a Stephen Peter Fitzgerald, Crumlin derivative of 3-phenylcyclohexanol.” J. Pharmacol. Exp. Ther. (GB); Paul John Innocenzi, Crumlin 1982, vol. 223, No. 2, pp. 516-523.* (GB); Philip Andrew Lowry, Crumlin Wild, “The Immunoassay Handbook.” Third Ed., Elsevier, 2005, (GB); Ivan Robert McConnell, pp. 255-256.* Crumlin (GB) Melvin et al., “A cannabinoid derived prototypical analgesic,” J. Med. Chem., 1984, vol. 27, No. 1, pp. 67-71.* Dresen, S. et al., “Monitoring of Herbal Mixtures Potentially (73) Assignee: Randox Laboratories Limited, Containing Synthetic Cannabinoids as Psychoactive Compounds.” Crumlin (GB) J. Mass. Spectrometry, 2010, pp. 1186-1194, vol. 45. Goodrow, M.H. et al., “Strategies for Immunoassay Hapten (*) Notice: Subject to any disclaimer, the term of this Design,” in Immunoanalysis of Agrochemicals; Nelson, J. et al., patent is extended or adjusted under 35 ACS Symposium Series, 1995, Chapter 9, pp. 119-139, vol. 586. U.S.C. 154(b) by 590 days. Hudson, S. et al., “Use of High-Resolution Accurate Mass Spec trometry to Detect Reported and Previously Unreported Can (21) Appl. No.: 13/585,630 nabinomimetics in Herbal High Products,” J. Anal. Toxicol., 2010, pp. 252-260, vol. 34. Huffman, J. et al., “1-Pentyl-3-phenylacetylindoles, a New Class of (22) Filed: Aug. -

Medicinal Chemistry Endeavors Around the Phytocannabinoids
CHEMISTRY & BIODIVERSITY – Vol. 4 (2007) 1707 REVIEW Medicinal Chemistry Endeavors around the Phytocannabinoids by Eric Stern and Didier M. Lambert* Drug Design and Discovery Center and Unite´ de Chimie pharmaceutique et de Radiopharmacie, Ecole de Pharmacie, Faculte´ de Me´decine, Universite´ catholique de Louvain, Avenue E. Mounier 73, U.C.L. 73.40, B-1200 Bruxelles (phone: þ3227647347; fax: þ3227647363; e-mail: [email protected]) Over the past 50 years, a considerable research in medicinal chemistry has been carried out around the natural constituents of Cannabis sativa L. Following the identification of D9-tetrahydrocannabinol (D9-THC) in 1964, critical chemical modifications, e.g., variation of the side chain at C(3) and the opening of the tricyclic scaffold, have led to the characterization of potent and cannabinoid receptor subtype-selective ligands. Those ligands that demonstrate high affinity for the cannabinoid receptors and good biological efficacy are still used as powerful pharmacological tools. This review summarizes past as well as recent developments in the structure–activity relationships of phytocannabinoids. 1. Introduction. – Despite the wide uses of preparations of the hemp Cannabis sativa L. during the History, the modern pharmacology of natural cannabinoids has been hampered by the slow progress in the elucidations of the chemical structures of its major components. Indeed, it is nowadays known that more than 70 compounds derived from a diterpene structure are present in the plant [1], and this fact may explain the difficulty to obtain pure chemical entities in the past. In addition, the medicinal research for more than a half century has been driven by the search for the components responsible for the psychoactive effects of cannabis, this era in the history of the chemical research on cannabinoids have been recently reviewed [2][3]. -

Article 22 Regulation for Restriction of Synthetic Drugs
ARTICLE 22 REGULATION FOR RESTRICTION OF SYNTHETIC DRUGS SECTION 22.1 AUTHORITY This regulation is promulgated under the authority granted to the Needham Board of Health under Massachusetts General Laws Chapter 111, Section 31 which states that “boards of health may make reasonable health regulations”. SECTION 22.2 PURPOSE The Needham Board of Health has found that synthetic marijuana, consisting of plant or other material treated with various chemicals or other synthetic substances not approved for human consumption, may be marketed and sold as herbal incense in the greater Boston area, although they are being used in the same manner and for the same purposes as scheduled drugs. In addition, the use of these products has become particularly popular among teens and young adults. Based on information and reports from hospitals, emergency room doctors, and police agencies, individuals who use these products experience dangerous side effects including convulsions, hallucinations, and dangerously elevated heart rates. This is evidence that synthetic marijuana products are harmful if inhaled or consumed, and present a significant public health danger. These synthetic compounds and others have a high potential for abuse and lack of any accepted medical use, these dangerous products, while not approved for human consumption, are marketed and sold in a form that allows for such consumption, putting at risk the individuals who come into contact with them. Therefore, the Needham Board of Health adopts this regulation for the purpose and with the intent to protect the public health and safety of the Town of Needham and its residents from the threat posed by the availability and use of synthetic marijuana, synthetic stimulants, synthetic hallucinogens, and other dangerous products by prohibiting persons from trafficking in, possessing, and using them within the town. -

Shlomi Cohen, Israel Manela, Eli More , Et Al. V. Pharmos Corp. , Et Al
UNITED STATES DISTRICT COURT DISTRICT OF NEW JERSEY _____________________________________ ) SHLOMI COHEN, ISRAEL MANELA, and ELI ) Case No. ____________ MORE, individually and on behalf of all others ) similarly situated, ) ) CLASS ACTION COMPLAINT ) Plaintiffs, ) ) JURY TRIAL DEMANDED v. ) ) HAIM AVIV; GAD RIESENFELD; and PHARMOS ) CORP., ) ) Defendants. ) _____________________________________ ) Plaintiffs, by undersigned counsel, for plaintiffs’ Class Action Complaint, allege the following upon personal knowledge as to plaintiffs and plaintiffs’ own acts, and upon information and belief based upon the investigation of plaintiffs’ attorneys as to all other matters. The investigation includes the review and analysis of public statements and publicly-filed documents of Pharmos Corp. ("Pharmos" or the "Company"), press releases, news articles and related literature. Plaintiffs believe that further substantial evidentiary support will exist for the allegations set forth below after a reasonable opportunity for discovery. SUMMARY OF ACTION 1. This is a securities class action on behalf of investors who purchased common stock of the Pharmos during the period from August 23, 2004 through December 17, 2004 (the "Class Period"). 2. Pharmos is a bio-pharmaceutical company that develops drugs to treat a range of neuro-inflammatory disorders. Pharmos’s main product, Dexanabinol, is a synthetic non- psychotropic cannabinoid for the treatment of traumatic brain injury. 3. Pharmos’s common stock is listed on the Nasdaq under the ticker symbol “PARS”; and as of November 1, 2004, Pharmos had 94.3 million shares outstanding. 4. Throughout the Class Period, defendants repeatedly touted Dexanabinol, causing Pharmos’ stock price to climb dramatically. In fact, Dexanabinol was simply ineffective in treating Traumatic Brain Injury, and defendants were aware of or recklessly disregarded evidence of that ineffectiveness. -

Long Term Cerebroprotective Effects of Dexanabinol in a Model of Focal Cerebral Ischemia Gil Lavieaa,Cbaa, , Angella Teichner , Esther Shohami , Haim Ovadia , Ronen R
Brain Research 901 (2001) 195±201 www.elsevier.com/locate/bres Research report Long term cerebroprotective effects of dexanabinol in a model of focal cerebral ischemia Gil Lavieaa,cbaa, , Angella Teichner , Esther Shohami , Haim Ovadia , Ronen R. Leker * aDepartment of Neurology, The Agnes Ginges Center for Human Neurogenetics, Hebrew University-Hadassah Medical School, Hadassah University Hospital, Ein Kerem, P.O. Box 12000, Jerusalem 91120, Israel bDepartment of Pharmacology, Hebrew University-Hadassah Medical School, Hadassah University Hospital, Jerusalem, Israel cDepartment of Neurological Sciences, University of Rome, La Sapienza, Rome, Italy Accepted 27 February 2001 Abstract In order to test the long-term cerebroprotective effects of dexanabinol, a synthetic non-competitive NMDA antagonist that also has anti-TNFa effects, spontaneously hypertensive rats underwent permanent middle cerebral artery occlusion (PMCAO). Rats were given vehicle or dexanabinol (4.5 mg/kg) 1, 3 or 6 h after PMCAO. The research consisted of 2 stages. In the short-term set of experiments animals (n55/group), were tested with a motor disability scale 24 h post PMCAO, then sacri®ced and the infarct volume was measured using 2,3,5-Triphenyltetrazolium chloride (TTC) staining. In the long-term set of experiments the rats (n57/group) were examined daily with a motor disability scale up to 30 days after PMCAO and then sacri®ced and infarct volumes were determined using TTC staining. Motor scores were signi®cantly improved in the dexanabinol treated rats (P,0.05 for all groups) at all the time points examined. Infarct volumes were signi®cantly reduced 24 h after PMCAO in the groups treated 1 or 3 h, but not 6 h after PMCAO compared with vehicle (Mean6S.D., 11.562.02, 1263.2 and 14.462.4% vs. -

How Is Spice Abused? What Are the Health Effects of Spice Abuse?
Spice “Spice” is used to describe a diverse What Are the Health Effects family of herbal mixtures marketed under of Spice Abuse? many names, including K2, fake marijuana, Presently, there are no studies on the effects Yucatan Fire, Skunk, Moon Rocks, and of Spice on human health or behavior. A others. These products contain dried, variety of mood and perceptual effects have shredded plant material and presumably, been described, and patients who have been chemical additives that are responsible for taken to Poison Control Centers in Texas their psychoactive (mind-altering) effects. report symptoms that include rapid heart While Spice products are labeled “not for rate, vomiting, agitation, confusion, and human consumption” they are marketed hallucinations. to people who are interested in herbal alternatives to marijuana (cannabis). Spice Public Health Concerns users report experiences similar to those produced by marijuana, and regular users Marketing labels often make unverified may experience withdrawal and addiction claims that Spice products contain up to 3.0 symptoms. grams of a natural psychoactive material taken from a variety of plants. While Spice Spice mixtures are sold in many countries products do contain dried plant material, in head shops, gas stations, and via the chemical analyses of seized spice mixtures Internet, although their sale and use are have revealed the presence of synthetic (or illegal throughout most European countries. designer) cannabinoid compounds.* These Easy access has likely contributed to Spice’s bind to the same cannabinoid receptors in the popularity. body as THC (delta-9-tetrahydrocannabinol), the primary psychoactive component of How Is Spice Abused? marijuana. -

(12) United States Patent (10) Patent No.: US 8.449,908 B2 Stinchcomb Et Al
USOO84499.08B2 (12) United States Patent (10) Patent No.: US 8.449,908 B2 Stinchcomb et al. (45) Date of Patent: May 28, 2013 (54) TRANSDERMAL DELIVERY OF 4,663,474 A 5, 1987 Urban CANNABIDOL 4,876,276 A 10, 1989 Mechoulam et al. 5,059,426 A * 10/1991 Chiang et al. ................. 424/449 5,223,262 A 6/1993 Kim et al. (75) Inventors: Audra L. Stinchcomb, Lexington, KY 5,254,346 A 10, 1993 Tucker et al. (US); Buchi N. Nalluri, Lexington, KY (US) (Continued) FOREIGN PATENT DOCUMENTS (73) Assignee: Alltranz, LLC, Lexington, KY (US) EP 0570920 A1 5, 1992 EP O656354 B1 12/1993 (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 (Continued) U.S.C. 154(b) by 1546 days. OTHER PUBLICATIONS (21) Appl. No.: 11/157,034 "Cannabinoid system as a potential target for drug development in the treatment of cardiovascular disease” by Mendizabal et al., Current (22) Filed: Jun. 20, 2005 Vascular Pharmacology, 2003, vol. 1, No. 3, 301-313.* (65) Prior Publication Data (Continued) US 2005/0266.061 A1 Dec. 1, 2005 Primary Examiner — Isis Ghali (74) Attorney, Agent, or Firm — Foley & Lardner LLP (51) Int. C. A6DF 3/00 (2006.01) (57) ABSTRACT A6DF 3/02 (2006.01) A 6LX 9/70 (2006.01) The present invention overcomes the problems associated A6IL 15/16 (2006.01) with existing drug delivery systems by delivering cannab U.S. C. inoids transdermally. Preferably, the cannabinoids are deliv (52) ered via an occlusive body (i.e., a patch) to alleviate harmful USPC ........................... -

Cannabinoids in Experimental Stroke: a Systematic Review
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repository@Nottingham CANNABINOIDS IN EXPERIMENTAL STROKE: A SYSTEMATIC REVIEW AND META-ANALYSIS Authors: Timothy J England (PhD), William H Hind (PhD), Nadiah A Rasid (BMedSci), Saoirse E O’Sullivan (PhD) Affiliations: Division of Medical Sciences & Graduate Entry Medicine, School of Medicine, University of Nottingham Running title: Cannabinoids and stroke: a systematic review Corresponding author: Timothy J England Division of Medical Sciences & Graduate Entry Medicine School of Medicine Faculty of Medicine & Health Sciences University of Nottingham Royal Derby Hospital Centre Uttoxeter Road, Derby DE22 3DT Tel: +44 1332 724668, Fax +44 1332 724697 Email: [email protected] Word Count (title page, abstract, main text, excluding references): 3957 Tables: 2 Figures: 5 [1] Abstract Cannabinoids (CB) show promise as neuroprotectants with some agents already licensed in humans for other conditions. We systematically reviewed CBs in pre-clinical stroke to guide further experimental protocols. We selected controlled studies assessing acute administration of CBs for experiment stroke, identified through systematic searches. Data were extracted on lesion volume, outcome and quality; analysed using random effects models; results are expressed as standardised mean difference (SMD) with 95% confidence intervals [CI]. 144 experiments (34 publications) assessed CBs on infarct volume in 1473 animals. CBs reduced infarct volume in transient (SMD -1.41, [95% CI -1.71,-1.11], p<0.00001) and permanent (-1.67 [-2.08,-1.27], p<0.00001) ischaemia and in all subclasses: endocannabinoids (-1.72 [-2.62,- 0.82], p=0.0002), CB1/CB2 ligands (-1.75 [-2.19,-1.31], p<0.00001), CB2 ligands (-1.65 [-2.09,-1.22], p<0.00001), cannabidiol (-1.20 [-1.63,-0.77], p<0.00001), Δ9-tetrahydrocannabinol (-1.43 [-2.01,-0.86], p<0.00001) and HU- 211 (-2.90 [-4.24,-1.56], p<0.0001). -

(12) Patent Application Publication (10) Pub. No.: US 2007/0104741 A1 Murty Et Al
US 20070 104741A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0104741 A1 Murty et al. (43) Pub. Date: May 10, 2007 (54) DELIVERY OF TETRAHYDROCANNABINOL Publication Classification (51) Int. Cl. (75) Inventors: Ram B. Murty, Lexington, KY (US); A6II 3 L/353 (2006.01) Santos B. Murty, Lexington, KY (US) A6IR 9/00 (2006.01) A6II 3 L/655 (2006.01) Correspondence Address: (52) U.S. Cl. ........................... 424/400: 514/454: 514/151 TOWNSEND & BANTA (57) ABSTRACT fo PORTFOLIO P A self-emulsifying drug delivery system to improve disso PO BOX S2OSO lution, stability, and bioavailability of drug compounds of MINNEAPOLIS, MN 55402 (US) dronabinol or other cannabinoids. The drug compound(s) are dissolved in an oily medium (e.g. triglycerides and/or (73) Assignee: Murty Pharmaceuticals, Inc., Lexing mixed glycerides and/or free fatty acids containing medium ton, KY and/or long chain Saturated, mono-unsaturated, and/or poly Appl. No.: 11/592,393 unsaturated free fatty acids) together with at least one (21) Surfactant. The Surfactant promotes self-emulsification, (22) Filed: Nov. 3, 2006 thereby promoting targeted chylomicron delivery and opti mal bioavailability to a mammalian intestinal lumen. A dosage form can optionally include co-solvents, anti-oxi Related U.S. Application Data dants, viscosity modifying agents, cytochrome P450 meta bolic inhibitors, P-GP efflux inhibitors, and amphiphilic/ (60) Provisional application No. 60/734,160, filed on Nov. non-amphiphilic solutes to induce semi-solid formation for 7, 2005. targeted release rates. -0-Formulation (i) -H-Formulation (ii) SO -A-Formulation (iii) de-Formulation (iv) 40 - -ie- Formulation (v) O Marketed Product O 20 40 60 8O Time (min) Patent Application Publication May 10, 2007 Sheet 1 of 3 US 2007/0104741 A1 120 100 - 8 O -0-Formulation (i) -HFormulation (ii) 6 O -A-Formulation (iii) -X-Formulation (iv) 4 O --Formulation (v) O Marketed Product 2 O O 20 40 60 80 Time (min) Fig. -

Glutamate NMDA Receptors in Pathophysiology And
® Postepy Hig Med Dosw (online), 2011; 65: 338-346 www.phmd.pl e-ISSN 1732-2693 Review Received: 2011.02.07 Accepted: 2011.05.11 Glutamate NMDA receptors in pathophysiology and Published: 2011.06.07 pharmacotherapy of selected nervous system diseases Rola receptorów NMDA w patofizjologii i farmakoterapii wybranych chorób układu nerwowego Łukasz Dobrek, Piotr Thor Department of Pathophysiology, Jagiellonian University Medical College Cracow, Poland Summary Glutamate is the basic excitatory neurotransmitter acting via N-methyl-D-aspartate receptors (NMDARs). It co-regulates many important physiological functions, including learning, memo- ry, and behaviour. An excess of glutamate, as well as NMDAR over-activity, produce pathologi- cal effects. Glutamate-related neurotoxicity is involved in the pathogenesis of many neurological conditions. This article briefly describes the role of the glutamate system in the pathophysiology of brain ischemia, selected neurodegenerative disorders, and schizophrenia. It also reviews the current and potential future status of agents targeting NMDARs in neuropsychopharmacology. Key words: glutamate • NMDA receptors (NMDARs) • excitotoxicity • NMDAR antagonists Streszczenie Glutaminian jest podstawowym neuroprzekaźnikiem pobudzającym, który działa na receptory NMDA. Związek ten jest współodpowiedzialny za regulowanie wielu ważnych fizjologicznych funkcji, wliczając w to uczenie się, pamięć i zachowanie. Nadmiar glutaminianu i nadaktywność receptorów NMDARs wywołuje patologiczne zmiany. Zjawisko neurotoksyczności -

House Committee Substitute for Senate Substitute for Senate Committee Substitute for Senate Bill No
SECOND REGULAR SESSION HOUSE COMMITTEE SUBSTITUTE FOR SENATE SUBSTITUTE FOR SENATE COMMITTEE SUBSTITUTE FOR SENATE BILL NO. 547 99TH GENERAL ASSEMBLY 5165H.07C D. ADAM CRUMBLISS, Chief Clerk AN ACT To repeal sections 195.010, 195.017, and 196.070, RSMo, and to enact in lieu thereof sixteen new sections relating to industrial hemp, with penalty provisions. Be it enacted by the General Assembly of the state of Missouri, as follows: Section A. Sections 195.010, 195.017, and 196.070, RSMo, are repealed and sixteen new 2 sections enacted in lieu thereof, to be known as sections 195.010, 195.017, 195.203, 195.740, 3 195.743, 195.746, 195.749, 195.752, 195.755, 195.756, 195.758, 195.764, 195.767, 195.770, 4 195.773, and 196.070, to read as follows: 195.010. The following words and phrases as used in this chapter and chapter 579, 2 unless the context otherwise requires, mean: 3 (1) "Addict", a person who habitually uses one or more controlled substances to such an 4 extent as to create a tolerance for such drugs, and who does not have a medical need for such 5 drugs, or who is so far addicted to the use of such drugs as to have lost the power of self-control 6 with reference to his or her addiction; 7 (2) "Administer", to apply a controlled substance, whether by injection, inhalation, 8 ingestion, or any other means, directly to the body of a patient or research subject by: 9 (a) A practitioner (or, in his or her presence, by his or her authorized agent); or 10 (b) The patient or research subject at the direction and in the presence of the practitioner; 11 (3) "Agent", an authorized person who acts on behalf of or at the direction of a 12 manufacturer, distributor, or dispenser. -

Cannabinoid CB Receptor Expression, Activation and Detection of Endogenous Ligand in Trabecular Meshwork and Ciliary Process
European Journal of Pharmacology 431Ž. 2001 277–286 www.elsevier.comrlocaterejphar Cannabinoid CB1 receptor expression, activation and detection of endogenous ligand in trabecular meshwork and ciliary process tissues W.D. Stamer a,b,c,), S.F. Golightly a, Y. Hosohata b, E.P. Ryan a, A.C. Porter d, E. Varga b, R.J. Noecker a, C.C. Felder d, H.I. Yamamura b,c,e,f a Department of Ophthalmology, The UniÕersity of Arizona Health Sciences Center, Tucson, AZ 85724, USA b Department of Pharmacology, The UniÕersity of Arizona Health Sciences Center, Tucson, AZ 85724, USA c Program in Neuroscience, The UniÕersity of Arizona Health Sciences Center, Tucson, AZ 85724, USA d Eli Lilly Research Laboratories, Neuroscience DiÕision, Indianapolis, IN 46285, USA e Department of Biochemistry, The UniÕersity of Arizona Health Sciences Center, Tucson, AZ 85724, USA f Department of Psychiatry, The UniÕersity of Arizona Health Sciences Center, Tucson, AZ 85724, USA Received 9 April 2001; received in revised form 28 August 2001; accepted 2 October 2001 Abstract Elevated intraocular pressure is the primary risk factor for glaucoma. Cannabinoids interact with molecular targets in the eye and lower intraocular pressure by an unknown mechanism. The purpose of the present study was to examine eye tissues for functional cannabinoid receptors of the neuronal, CB1 class, and an endogenous ligand, anandamide. The trabecular meshwork and ciliary processes are the primary structures of the eye that contribute to intraocular pressure and thus were our focus. Total RNA, frozen sections, cellular membranes and primary cultures of cells were prepared from both bovine and cadaveric human tissues.