Phenotypic Image Analysis Software Tools for Exploring and Understanding Big Image Data from Cell-Based Assays
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Management of Large Sets of Image Data Capture, Databases, Image Processing, Storage, Visualization Karol Kozak
Management of large sets of image data Capture, Databases, Image Processing, Storage, Visualization Karol Kozak Download free books at Karol Kozak Management of large sets of image data Capture, Databases, Image Processing, Storage, Visualization Download free eBooks at bookboon.com 2 Management of large sets of image data: Capture, Databases, Image Processing, Storage, Visualization 1st edition © 2014 Karol Kozak & bookboon.com ISBN 978-87-403-0726-9 Download free eBooks at bookboon.com 3 Management of large sets of image data Contents Contents 1 Digital image 6 2 History of digital imaging 10 3 Amount of produced images – is it danger? 18 4 Digital image and privacy 20 5 Digital cameras 27 5.1 Methods of image capture 31 6 Image formats 33 7 Image Metadata – data about data 39 8 Interactive visualization (IV) 44 9 Basic of image processing 49 Download free eBooks at bookboon.com 4 Click on the ad to read more Management of large sets of image data Contents 10 Image Processing software 62 11 Image management and image databases 79 12 Operating system (os) and images 97 13 Graphics processing unit (GPU) 100 14 Storage and archive 101 15 Images in different disciplines 109 15.1 Microscopy 109 360° 15.2 Medical imaging 114 15.3 Astronomical images 117 15.4 Industrial imaging 360° 118 thinking. 16 Selection of best digital images 120 References: thinking. 124 360° thinking . 360° thinking. Discover the truth at www.deloitte.ca/careers Discover the truth at www.deloitte.ca/careers © Deloitte & Touche LLP and affiliated entities. Discover the truth at www.deloitte.ca/careers © Deloitte & Touche LLP and affiliated entities. -

10 Oriented Principal Component Analysis for Feature Extraction
10 Oriented Principal Component Analysis for Feature Extraction Abstract- Principal Components Analysis (PCA) is a popular unsupervised learning technique that is often used in pattern recognition for feature extraction. Some variants have been proposed for supervising PCA to include class information, which are usually based on heuristics. A more principled approach is presented in this paper. Suppose that we have a pattern recogniser composed of a linear network for feature extraction and a classifier. Instead of designing each module separately, we perform global training where both learning systems cooperate in order to find those components (Oriented PCs) useful for classification. Experimental results, using artificial and real data, show the potential of the proposed learning system for classification. Since the linear neural network finds the optimal directions to better discriminate classes, the global-trained pattern recogniser needs less Oriented PCs (OPCs) than PCs so the classifier’s complexity (e.g. capacity) is lower. In this way, the global system benefits from a better generalisation performance. Index Terms- Oriented Principal Components Analysis, Principal Components Neural Networks, Co-operative Learning, Learning to Learn Algorithms, Feature Extraction, Online gradient descent, Pattern Recognition, Compression. Abbreviations- PCA- Principal Components Analysis, OPC- Oriented Principal Components, PCNN- Principal Components Neural Networks. 1. Introduction In classification, observations belong to different classes. Based on some prior knowledge about the problem and on the training set, a pattern recogniser is constructed for assigning future observations to one of the existing classes. The typical method of building this system consists in dividing it into two main blocks that are usually trained separately: the feature extractor and the classifier. -
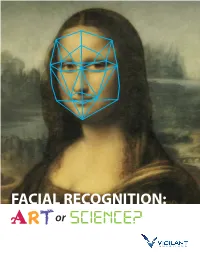
FACIAL RECOGNITION: a Or FACIALRT RECOGNITION: a Or RT Facial Recognition – Art Or Science?
FACIAL RECOGNITION: A or FACIALRT RECOGNITION: A or RT Facial Recognition – Art or Science? Both the public and law enforcement often misunderstand facial recognition technology. Hollywood would have you believe that with facial recognition technology, law enforcement can identify an individual from an image, while also accessing all of their personal information. The “Big Brother” narrative makes good television, but it vastly misrepresents how law enforcement agencies actually use facial recognition. Additionally, crime dramas like “CSI,” and its endless spinoffs, spin the narrative that law enforcement easily uses facial recognition Roger Rodriguez joined to generate matches. In reality, the facial recognition process Vigilant Solutions after requires a great deal of manual, human analysis and an image of serving over 20 years a certain quality to make a possible match. To date, the quality with the NYPD, where he threshold for images has been hard to reach and has often spearheaded the NYPD’s first frustrated law enforcement looking to generate effective leads. dedicated facial recognition unit. The unit has conducted Think of facial recognition as the 21st-century evolution of the more than 8,500 facial sketch artist. It holds the promise to be much more accurate, recognition investigations, but in the end it is still just the source of a lead that needs to be with over 3,000 possible matches and approximately verified and followed up by intelligent police work. 2,000 arrests. Roger’s enhancement techniques are Today, innovative facial now recognized worldwide recognition technology and have changed law techniques make it possible to enforcement’s approach generate investigative leads to the utilization of facial regardless of image quality. -

Bioimage Analysis Tools
Bioimage Analysis Tools Kota Miura, Sébastien Tosi, Christoph Möhl, Chong Zhang, Perrine Paul-Gilloteaux, Ulrike Schulze, Simon Norrelykke, Christian Tischer, Thomas Pengo To cite this version: Kota Miura, Sébastien Tosi, Christoph Möhl, Chong Zhang, Perrine Paul-Gilloteaux, et al.. Bioimage Analysis Tools. Kota Miura. Bioimage Data Analysis, Wiley-VCH, 2016, 978-3-527-80092-6. hal- 02910986 HAL Id: hal-02910986 https://hal.archives-ouvertes.fr/hal-02910986 Submitted on 3 Aug 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 2 Bioimage Analysis Tools 1 2 3 4 5 6 Kota Miura, Sébastien Tosi, Christoph Möhl, Chong Zhang, Perrine Pau/-Gilloteaux, - Ulrike Schulze,7 Simon F. Nerrelykke,8 Christian Tischer,9 and Thomas Penqo'" 1 European Molecular Biology Laboratory, Meyerhofstraße 1, 69117 Heidelberg, Germany National Institute of Basic Biology, Okazaki, 444-8585, Japan 2/nstitute for Research in Biomedicine ORB Barcelona), Advanced Digital Microscopy, Parc Científic de Barcelona, dBaldiri Reixac 1 O, 08028 Barcelona, Spain 3German Center of Neurodegenerative -

Other Departments and Institutes Courses 1
Other Departments and Institutes Courses 1 Other Departments and Institutes Courses About Course Numbers: 02-250 Introduction to Computational Biology Each Carnegie Mellon course number begins with a two-digit prefix that Spring: 12 units designates the department offering the course (i.e., 76-xxx courses are This class provides a general introduction to computational tools for biology. offered by the Department of English). Although each department maintains The course is divided into two halves. The first half covers computational its own course numbering practices, typically, the first digit after the prefix molecular biology and genomics. It examines important sources of biological indicates the class level: xx-1xx courses are freshmen-level, xx-2xx courses data, how they are archived and made available to researchers, and what are sophomore level, etc. Depending on the department, xx-6xx courses computational tools are available to use them effectively in research. may be either undergraduate senior-level or graduate-level, and xx-7xx In the process, it covers basic concepts in statistics, mathematics, and courses and higher are graduate-level. Consult the Schedule of Classes computer science needed to effectively use these resources and understand (https://enr-apps.as.cmu.edu/open/SOC/SOCServlet/) each semester for their results. Specific topics covered include sequence data, searching course offerings and for any necessary pre-requisites or co-requisites. and alignment, structural data, genome sequencing, genome analysis, genetic variation, gene and protein expression, and biological networks and pathways. The second half covers computational cell biology, including biological modeling and image analysis. It includes homework requiring Computational Biology Courses modification of scripts to perform computational analyses. -

Cellanimation: an Open Source MATLAB Framework for Microscopy Assays Walter Georgescu1,2,3,∗, John P
Vol. 28 no. 1 2012, pages 138–139 BIOINFORMATICS APPLICATIONS NOTE doi:10.1093/bioinformatics/btr633 Systems biology Advance Access publication November 24, 2011 CellAnimation: an open source MATLAB framework for microscopy assays Walter Georgescu1,2,3,∗, John P. Wikswo1,2,3,4,5 and Vito Quaranta1,3,6 1Vanderbilt Institute for Integrative Biosystems Research and Education, 2Department of Biomedical Engineering, Vanderbilt University, 3Center for Cancer Systems Biology at Vanderbilt, Vanderbilt University Medical Center, Nashville, TN, USA, 4Department of Molecular Physiology and Biophysics, 5Department of Physics and Astronomy, Vanderbilt University and 6Department of Cancer Biology, Vanderbilt University Medical Center, Nashville, TN, USA Associate Editor: Jonathan Wren ABSTRACT 1 INTRODUCTION Motivation: Advances in microscopy technology have led to At present there are a number of microscopy applications on the the creation of high-throughput microscopes that are capable of market, both open-source and commercial, and addressing both generating several hundred gigabytes of images in a few days. very specific and broader microscopy needs. In general, commercial Analyzing such wealth of data manually is nearly impossible and software packages such as Imaris® and MetaMorph® tend to requires an automated approach. There are at present a number include more complete sets of algorithms, covering different kinds of open-source and commercial software packages that allow the of experimental setups, at the cost of ease of customization and user -

Abstractband
Volume 23 · Supplement 1 · September 2013 Clinical Neuroradiology Official Journal of the German, Austrian, and Swiss Societies of Neuroradiology Abstracts zur 48. Jahrestagung der Deutschen Gesellschaft für Neuroradiologie Gemeinsame Jahrestagung der DGNR und ÖGNR 10.–12. Oktober 2013, Gürzenich, Köln www.cnr.springer.de Clinical Neuroradiology Official Journal of the German, Austrian, and Swiss Societies of Neuroradiology Editors C. Ozdoba L. Solymosi Bern, Switzerland (Editor-in-Chief, responsible) ([email protected]) Department of Neuroradiology H. Urbach University Würzburg Freiburg, Germany Josef-Schneider-Straße 11 ([email protected]) 97080 Würzburg Germany ([email protected]) Published on behalf of the German Society of Neuroradiology, M. Bendszus president: O. Jansen, Heidelberg, Germany the Austrian Society of Neuroradiology, ([email protected]) president: J. Trenkler, and the Swiss Society of Neuroradiology, T. Krings president: L. Remonda. Toronto, ON, Canada ([email protected]) Clin Neuroradiol 2013 · No. 1 © Springer-Verlag ABC jobcenter-medizin.de Clin Neuroradiol DOI 10.1007/s00062-013-0248-4 ABSTRACTS 48. Jahrestagung der Deutschen Gesellschaft für Neuroradiologie Gemeinsame Jahrestagung der DGNR und ÖGNR 10.–12. Oktober 2013 Gürzenich, Köln Kongresspräsidenten Prof. Dr. Arnd Dörfler Prim. Dr. Johannes Trenkler Erlangen Wien Dieses Supplement wurde von der Deutschen Gesellschaft für Neuroradiologie finanziert. Inhaltsverzeichnis Grußwort ............................................................................................................................................................................ -

Feature Selection/Extraction
Feature Selection/Extraction Dimensionality Reduction Feature Selection/Extraction • Solution to a number of problems in Pattern Recognition can be achieved by choosing a better feature space. • Problems and Solutions: – Curse of Dimensionality: • #examples needed to train classifier function grows exponentially with #dimensions. • Overfitting and Generalization performance – What features best characterize class? • What words best characterize a document class • Subregions characterize protein function? – What features critical for performance? – Subregions characterize protein function? – Inefficiency • Reduced complexity and run-time – Can’t Visualize • Allows ‘intuiting’ the nature of the problem solution. Curse of Dimensionality Same Number of examples Fill more of the available space When the dimensionality is low Selection vs. Extraction • Two general approaches for dimensionality reduction – Feature extraction: Transforming the existing features into a lower dimensional space – Feature selection: Selecting a subset of the existing features without a transformation • Feature extraction – PCA – LDA (Fisher’s) – Nonlinear PCA (kernel, other varieties – 1st layer of many networks Feature selection ( Feature Subset Selection ) Although FS is a special case of feature extraction, in practice quite different – FSS searches for a subset that minimizes some cost function (e.g. test error) – FSS has a unique set of methodologies Feature Subset Selection Definition Given a feature set x={xi | i=1…N} find a subset xM ={xi1, xi2, …, xiM}, with M<N, that optimizes an objective function J(Y), e.g. P(correct classification) Why Feature Selection? • Why not use the more general feature extraction methods? Feature Selection is necessary in a number of situations • Features may be expensive to obtain • Want to extract meaningful rules from your classifier • When you transform or project, measurement units (length, weight, etc.) are lost • Features may not be numeric (e.g. -

Feature Extraction (PCA & LDA)
Feature Extraction (PCA & LDA) CE-725: Statistical Pattern Recognition Sharif University of Technology Spring 2013 Soleymani Outline What is feature extraction? Feature extraction algorithms Linear Methods Unsupervised: Principal Component Analysis (PCA) Also known as Karhonen-Loeve (KL) transform Supervised: Linear Discriminant Analysis (LDA) Also known as Fisher’s Discriminant Analysis (FDA) 2 Dimensionality Reduction: Feature Selection vs. Feature Extraction Feature selection Select a subset of a given feature set Feature extraction (e.g., PCA, LDA) A linear or non-linear transform on the original feature space ⋮ ⋮ → ⋮ → ⋮ = ⋮ Feature Selection Feature ( <) Extraction 3 Feature Extraction Mapping of the original data onto a lower-dimensional space Criterion for feature extraction can be different based on problem settings Unsupervised task: minimize the information loss (reconstruction error) Supervised task: maximize the class discrimination on the projected space In the previous lecture, we talked about feature selection: Feature selection can be considered as a special form of feature extraction (only a subset of the original features are used). Example: 0100 X N 4 XX' 0010 X' N 2 Second and thirth features are selected 4 Feature Extraction Unsupervised feature extraction: () () A mapping : ℝ →ℝ ⋯ Or = ⋮⋱⋮ Feature Extraction only the transformed data () () () () ⋯ ′ ⋯′ = ⋮⋱⋮ () () ′ ⋯′ Supervised feature extraction: () () A mapping : ℝ →ℝ ⋯ Or = ⋮⋱⋮ Feature Extraction only the transformed -

Feature Extraction for Image Selection Using Machine Learning
Master of Science Thesis in Electrical Engineering Department of Electrical Engineering, Linköping University, 2017 Feature extraction for image selection using machine learning Matilda Lorentzon Master of Science Thesis in Electrical Engineering Feature extraction for image selection using machine learning Matilda Lorentzon LiTH-ISY-EX--17/5097--SE Supervisor: Marcus Wallenberg ISY, Linköping University Tina Erlandsson Saab Aeronautics Examiner: Lasse Alfredsson ISY, Linköping University Computer Vision Laboratory Department of Electrical Engineering Linköping University SE-581 83 Linköping, Sweden Copyright © 2017 Matilda Lorentzon Abstract During flights with manned or unmanned aircraft, continuous recording can result in a very high number of images to analyze and evaluate. To simplify image analysis and to minimize data link usage, appropriate images should be suggested for transfer and further analysis. This thesis investigates features used for selection of images worthy of further analysis using machine learning. The selection is done based on the criteria of having good quality, salient content and being unique compared to the other selected images. The investigation is approached by implementing two binary classifications, one regard- ing content and one regarding quality. The classifications are made using support vector machines. For each of the classifications three feature extraction methods are performed and the results are compared against each other. The feature extraction methods used are histograms of oriented gradients, features from the discrete cosine transform domain and features extracted from a pre-trained convolutional neural network. The images classified as both good and salient are then clustered based on similarity measures retrieved using color coherence vectors. One image from each cluster is retrieved and those are the result- ing images from the image selection. -

An Introduction to Image Analysis Using Imagej
An introduction to image analysis using ImageJ Mark Willett, Imaging and Microscopy Centre, Biological Sciences, University of Southampton. Pete Johnson, Biophotonics lab, Institute for Life Sciences University of Southampton. 1 “Raw Images, regardless of their aesthetics, are generally qualitative and therefore may have limited scientific use”. “We may need to apply quantitative methods to extrapolate meaningful information from images”. 2 Examples of statistics that can be extracted from image sets . Intensities (FRET, channel intensity ratios, target expression levels, phosphorylation etc). Object counts e.g. Number of cells or intracellular foci in an image. Branch counts and orientations in branching structures. Polarisations and directionality . Colocalisation of markers between channels that may be suggestive of structure or multiple target interactions. Object Clustering . Object Tracking in live imaging data. 3 Regardless of the image analysis software package or code that you use….. • ImageJ, Fiji, Matlab, Volocity and IMARIS apps. • Java and Python coding languages. ….image analysis comprises of a workflow of predefined functions which can be native, user programmed, downloaded as plugins or even used between apps. This is much like a flow diagram or computer code. 4 Here’s one example of an image analysis workflow: Apply ROI Choose Make Acquisition Processing to original measurement measurements image type(s) Thresholding Save to ROI manager Make binary mask Make ROI from binary using “Create selection” Calculate x̄, Repeat n Chart data SD, TTEST times and interpret and Δ 5 A few example Functions that can inserted into an image analysis workflow. You can mix and match them to achieve the analysis that you want. -

A Review on Image Segmentation Clustering Algorithms Devarshi Naik , Pinal Shah
Devarshi Naik et al, / (IJCSIT) International Journal of Computer Science and Information Technologies, Vol. 5 (3) , 2014, 3289 - 3293 A Review on Image Segmentation Clustering Algorithms Devarshi Naik , Pinal Shah Department of Information Technology, Charusat University CSPIT, Changa, di.Anand, GJ,India Abstract— Clustering attempts to discover the set of consequential groups where those within each group are more closely related to one another than the others assigned to different groups. Image segmentation is one of the most important precursors for image processing–based applications and has a crucial impact on the overall performance of the developed systems. Robust segmentation has been the subject of research for many years, but till now published work indicates that most of the developed image segmentation algorithms have been designed in conjunction with particular applications. The aim of the segmentation process consists of dividing the input image into several disjoint regions with similar characteristics such as colour and texture. Keywords— Clustering, K-means, Fuzzy C-means, Expectation Maximization, Self organizing map, Hierarchical, Graph Theoretic approach. Fig. 1 Similar data points grouped together into clusters I. INTRODUCTION II. SEGMENTATION ALGORITHMS Images are considered as one of the most important Image segmentation is the first step in image analysis medium of conveying information. The main idea of the and pattern recognition. It is a critical and essential image segmentation is to group pixels in homogeneous component of image analysis system, is one of the most regions and the usual approach to do this is by ‘common difficult tasks in image processing, and determines the feature. Features can be represented by the space of colour, quality of the final result of analysis.