The Use of Multi‑Species, Multi‑Scale Habitat Suitability Models
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
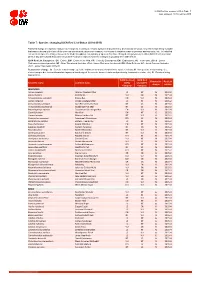
Table 7: Species Changing IUCN Red List Status (2014-2015)
IUCN Red List version 2015.4: Table 7 Last Updated: 19 November 2015 Table 7: Species changing IUCN Red List Status (2014-2015) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2014 (IUCN Red List version 2014.3) and 2015 (IUCN Red List version 2015-4) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2014) List (2015) change version Category Category MAMMALS Aonyx capensis African Clawless Otter LC NT N 2015-2 Ailurus fulgens Red Panda VU EN N 2015-4 -

First Record of Hose's Civet Diplogale Hosei from Indonesia
First record of Hose’s Civet Diplogale hosei from Indonesia, and records of other carnivores in the Schwaner Mountains, Central Kalimantan, Indonesia Hiromitsu SAMEJIMA1 and Gono SEMIADI2 Abstract One of the least-recorded carnivores in Borneo, Hose’s Civet Diplogale hosei , was filmed twice in a logging concession, the Katingan–Seruyan Block of Sari Bumi Kusuma Corporation, in the Schwaner Mountains, upper Seruyan River catchment, Central Kalimantan. This, the first record of this species in Indonesia, is about 500 km southwest of its previously known distribution (northern Borneo: Sarawak, Sabah and Brunei). Filmed at 325The m a.s.l., IUCN these Red List records of Threatened are below Species the previously known altitudinal range (450–1,800Prionailurus m). This preliminary planiceps survey forPardofelis medium badia and large and Otter mammals, Civet Cynogalerunning 100bennettii camera-traps in 10 plots for one (Bandedyear, identified Civet Hemigalus in this concession derbyanus 17 carnivores, Arctictis including, binturong on Neofelis diardi, three Endangered Pardofe species- lis(Flat-headed marmorata Cat and Sun Bear Helarctos malayanus, Bay Cat . ) and six Vulnerable species , Binturong , Sunda Clouded Leopard , Marbled Cat Keywords Cynogale bennettii, as well, Pardofelis as Hose’s badia Civet), Prionailurus planiceps Catatan: PertamaBorneo, camera-trapping, mengenai Musang Gunung Diplogale hosei di Indonesia, serta, sustainable karnivora forest management lainnya di daerah Pegunungan Schwaner, Kalimantan Tengah Abstrak Diplogale hosei Salah satu jenis karnivora yang jarang dijumpai di Borneo, Musang Gunung, , telah terekam dua kali di daerah- konsesi hutan Blok Katingan–Seruyan- PT. Sari Bumi Kusuma, Pegunungan Schwaner, di sekitar hulu Sungai Seruya, Kalimantan Tengah. Ini merupakan catatan pertama spesies tersebut terdapat di Indonesia, sekitar 500 km dari batas sebaran yang diketa hui saat ini (Sarawak, Sabah, Brunei). -

1 the Origin and Evolution of the Domestic Cat
1 The Origin and Evolution of the Domestic Cat There are approximately 40 different species of the cat family, classification Felidae (Table 1.1), all of which are descended from a leopard-like predator Pseudaelurus that existed in South-east Asia around 11 million years ago (O’Brien and Johnson, 2007). Other than the domestic cat, the most well known of the Felidae are the big cats such as lions, tigers and panthers, sub-classification Panthera. But the cat family also includes a large number of small cats, including a group commonly known as the wildcats, sub-classification Felis silvestris (Table 1.2). Physical similarity suggests that the domestic cat (Felis silvestris catus) originally derived from one or more than one of these small wildcats. DNA examination shows that it is most closely related to the African wildcat (Felis silvestris lybica), which has almost identical DNA, indicating that the African wildcat is the domestic cat’s primary ancestor (Lipinski et al., 2008). The African Wildcat The African wildcat is still in existence today and is a solitary and highly territorial animal indigenous to areas of North Africa and the Near East, the region where domestication of the cat is believed to have first taken place (Driscoll et al., 2007; Faure and Kitchener, 2009). It is primarily a nocturnal hunter that preys mainly on rodents but it will also eat insects, reptiles and other mammals including the young of small antelopes. Also known as the Arabian or North African wildcat, it is similar in appearance to a domestic tabby, with a striped grey/sandy-coloured coat, but is slightly larger and with longer legs (Fig. -

SECTION ONE: Background: Supply & Sources of Bear Products
SECTION ONE: Background: Supply & Sources of Bear Products Historical Perspective to the Bear Trade 16 Bear Farming 28 Profiles of Chinese bear farms 47 Current Restrictions on International Trade: CITES (Convention on International Trade in Endangered Species) 59 World Society for the Protection of Animals The Bear Bile Business 15 Historical Perspective to the Bear Trade Victor Watkins Traditonal Chinese Medicine and the growth of the modern trade in bear products The use of herbs to cure illness can be traced back over 4,000 years in China. The earliest medicinal literature (Shen-nong Ben Cao) dates back to 482 BC and records 365 types of medicinal issues. One of the most famous Chinese herbals, (Ben Cao Gang Mu) was written by Li Shi-zhen during the Ming dynasty (1590). This work lists 1,892 types of herbs used as medicine. In the above mentioned literature, animal ingredients make up less than 10% of the medicinal ingredients, and the majority of those animal parts are insects. There is very little use of mammal body parts listed in these early Chinese traditional medicines1. The use of bear parts in medicines in China dates back over 3,000 years. Medicinal uses for bear gall bladder first appeared in writing in the seventh century A.D. in the Materia Medica of Medicinal Properties2. The use of bear bile has since spread to other Asian countries such as Korea and Japan where it has been adopted for use in local traditional medicines. Plant and animal products which are selected for use in Chinese medicine are classified according to their properties. -

Heart Rate During Hyperphagia Differs Between Two Bear Species
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Brage INN Physiology Heart rate during hyperphagia differs royalsocietypublishing.org/journal/rsbl between two bear species Boris Fuchs1,†, Koji Yamazaki2,†, Alina L. Evans1, Toshio Tsubota3, Shinsuke Koike4,5, Tomoko Naganuma5 and Jon M. Arnemo1,6 Research 1Department of Forestry and Wildlife Management, Faculty of Applied Ecology and Agricultural Sciences, Cite this article: Fuchs B, Yamazaki K, Evans Inland Norway University of Applied Sciences, Campus Evenstad, 2418 Elverum, Norway 2Department of Forest Science, Tokyo University of Agriculture, 1-1-1 Sakuragaoka, Setagaya-Ku, Tokyo, Japan AL, Tsubota T, Koike S, Naganuma T, Arnemo 3Department of Environmental Veterinary Sciences, Faculty of Veterinary Medicine, Hokkaido University, Kita18, JM. 2019 Heart rate during hyperphagia differs Nishi9, Kita-Ku, Sapporo, Hokkaido, Japan between two bear species. Biol. Lett. 15: 4Institute of Global Innovation Research, and 5United Graduate School of Agricultural Science, Tokyo University 20180681. of Agriculture and Technology, 3-5-8 Saiwai, Fuchu-city, Tokyo, Japan 6Department of Wildlife, Fish and Environmental Studies, Faculty of Forest Sciences, Swedish University of http://dx.doi.org/10.1098/rsbl.2018.0681 Agricultural Sciences, 901 83, Umea˚, Sweden BF, 0000-0003-3412-3490; ALE, 0000-0003-0513-4887 Received: 28 September 2018 Hyperphagia is a critical part of the yearly cycle of bears when they gain fat reserves before entering hibernation. We used heart rate as a proxy to com- Accepted: 17 December 2018 pare the metabolic rate between the Asian black bear (Ursus thibetanus)in Japan and the Eurasian brown bear (Ursus arctos) in Sweden from summer into hibernation. -

Marbled Cat Pardofelis Marmorata at Virachey National Park, Ratanakiri, Cambodia
SEAVR 2016: 72-74 ISSN : 2424-8525 Date of publication: 13 May 2016. Hosted online by ecologyasia.com Marbled Cat Pardofelis marmorata at Virachey National Park, Ratanakiri, Cambodia Gregory Edward McCann greg.mccann1 @ gmail.com Observer: Gregory Edward McCann (camera trap installer) Photographs by: Habitat ID (www.habitatid.org) & Virachey National Park staff. Subject identified by: Gregory Edward McCann. Location: Virachey National Park, Ratanakiri province, Cambodia. Elevation: 1,455 metres. Habitat: Bamboo-dominated forest on mountain ridge. Date and time: 24 January 2016, 13:29 hrs. Identity of subject: Marbled Cat, Pardofelis marmorata (Mammalia: Carnivora: Felidae). Description of record: A lone Marbled Cat was photographed by camera trap in Virachey National Park (VNP), on the summit of Phnom Haling, one of the highest mountain ridges in northeast Cambodia, in an area dominated by bamboo (Figs. 1 and 2.). Fig. 1 : Full frame camera trap image. © Gregory Edward McCann 72 Fig. 2 : Cropped camera trap image. © Gregory Edward McCann Remarks: The subject is identified as a Marbled Cat Pardofelis marmorata based on its fur patterning, which includes large, dark blotches on its limbs, and its stocky shape. In addition the 'cloudy' pattern of lines on its back distinguishes it from the larger Clouded Leopard Neofelis nebulosa. It appears to be an adult and, based on its posture and the condition of its coat, it seems to be in healthy condition. Preliminary results of an on-going camera trapping program in VNP (which commenced in January 2014) have, as of March 2016, also resulted in 13 other trigger events of Marbled Cat, from seven different camera stations. -

Asiatic Golden Cat in Thailand Population & Habitat Viability Assessment
Asiatic Golden Cat in Thailand Population & Habitat Viability Assessment Chonburi, Thailand 5 - 7 September 2005 FINAL REPORT Photos courtesy of Ron Tilson, Sumatran Tiger Conservation Program (golden cat) and Kathy Traylor-Holzer, CBSG (habitat). A contribution of the IUCN/SSC Conservation Breeding Specialist Group. Traylor-Holzer, K., D. Reed, L. Tumbelaka, N. Andayani, C. Yeong, D. Ngoprasert, and P. Duengkae (eds.). 2005. Asiatic Golden Cat in Thailand Population and Habitat Viability Assessment: Final Report. IUCN/SSC Conservation Breeding Specialist Group, Apple Valley, MN. IUCN encourages meetings, workshops and other fora for the consideration and analysis of issues related to conservation, and believes that reports of these meetings are most useful when broadly disseminated. The opinions and views expressed by the authors may not necessarily reflect the formal policies of IUCN, its Commissions, its Secretariat or its members. The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. © Copyright CBSG 2005 Additional copies of Asiatic Golden Cat of Thailand Population and Habitat Viability Assessment can be ordered through the IUCN/SSC Conservation Breeding Specialist Group, 12101 Johnny Cake Ridge Road, Apple Valley, MN 55124, USA (www.cbsg.org). The CBSG Conservation Council These generous contributors make the work of CBSG possible Providers $50,000 and above Paignton Zoo Emporia Zoo Parco Natura Viva - Italy Laurie Bingaman Lackey Chicago Zoological Society Perth Zoo Lee Richardson Zoo -Chairman Sponsor Philadelphia Zoo Montgomery Zoo SeaWorld, Inc. -

Assessing the Distribution and Habitat Use of Four Felid Species in Bukit Barisan Selatan National Park, Sumatra, Indonesia
Global Ecology and Conservation 3 (2015) 210–221 Contents lists available at ScienceDirect Global Ecology and Conservation journal homepage: www.elsevier.com/locate/gecco Original research article Assessing the distribution and habitat use of four felid species in Bukit Barisan Selatan National Park, Sumatra, Indonesia Jennifer L. McCarthy a,∗, Hariyo T. Wibisono b,c, Kyle P. McCarthy b, Todd K. Fuller a, Noviar Andayani c a Department of Environmental Conservation, University of Massachusetts Amherst, 160 Holdsworth Way, Amherst, MA 01003, USA b Department of Entomology and Wildlife Ecology, University of Delaware, 248B Townsend Hall, Newark, DE 19716, USA c Wildlife Conservation Society—Indonesia Program, Jalan Atletik No. 8, Tanah Sareal, Bogor 16161, Indonesia article info a b s t r a c t Article history: There have been few targeted studies of small felids in Sumatra and there is little in- Received 24 October 2014 formation on their ecology. As a result there are no specific management plans for the Received in revised form 17 November species on Sumatra. We examined data from a long-term camera trapping effort, and used 2014 Maximum Entropy Modeling to assess the habitat use and distribution of Sunda clouded Accepted 17 November 2014 leopards (Neofelis diardi), Asiatic golden cats (Pardofelis temminckii), leopard cats (Pri- Available online 21 November 2014 onailurus bengalensis), and marbled cats (Pardofelis marmorata) in Bukit Barisan Sela- tan National Park. Over a period of 34,166 trap nights there were low photo rates (photo Keywords: Species distribution modeling events/100 trap nights) for all species; 0.30 for golden cats, 0.15 for clouded leopards, 0.10 Neofelis diardi for marbled cats, and 0.08 for leopard cats. -

Small Carnivores
SMALL CARNIVORES IN TINJURE-MILKE-JALJALE, EASTERN NEPAL The content of this booklet can be used freely with permission for any conservation and education purpose. However we would be extremely happy to get a hard copy or soft copy of the document you have used it for. For further information: Friends of Nature Kathmandu, Nepal P.O. Box: 23491 Email: [email protected], Website: www.fonnepal.org Facebook: www.facebook.com/fonnepal2005 First Published: April, 2018 Photographs: Friends of Nature (FON), Jeevan Rai, Zaharil Dzulkafly, www.pixabay/ werner22brigitte Design: Roshan Bhandari Financial support: Rufford Small Grants, UK Authors: Jeevan Rai, Kaushal Yadav, Yadav Ghimirey, Som GC, Raju Acharya, Kamal Thapa, Laxman Prasad Poudyal and Nitesh Singh ISBN: 978-9937-0-4059-4 Acknowledgements: We are grateful to Zaharil Dzulkafly for his photographs of Marbled Cat, and Andrew Hamilton and Wildscreen for helping us get them. We are grateful to www.pixabay/werner22brigitte for giving us Binturong’s photograph. We thank Bidhan Adhikary, Thomas Robertson, and Humayra Mahmud for reviewing and providing their valuable suggestions. Preferred Citation: Rai, J., Yadav, K., Ghimirey, Y., GC, S., Acharya, R., Thapa, K., Poudyal, L.P., and Singh, N. 2018. Small Carnivores in Tinjure-Milke -Jaljale, Eastern Nepal. Friends of Nature, Nepal and Rufford Small Grants, UK. Small Carnivores in Tinjure-Milke-Jaljale, Eastern Nepal Why Protect Small Carnivore! Small carnivores are an integral part of our ecosystem. Except for a few charismatic species such as Red Panda, a general lack of research and conservation has created an information gap about them. I am optimistic that this booklet will, in a small way, be the starting journey of filling these gaps in our knowledge bank of small carnivore in Nepal. -

Photographic Evidence of Marbled Cat from Dampa Ti- Ger Reserve, Mizoram, India
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/312619825 Photographic evidence of marbled cat from Dampa Ti- ger Reserve, Mizoram, India Article · January 2017 CITATIONS READS 0 88 3 authors, including: Janmejay Sethy Sushanto Gouda Amity University Amity University 43 PUBLICATIONS 22 CITATIONS 31 PUBLICATIONS 112 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Proteomic and genomic responses of plants to nutritional stress View project Status and Distribution of Malayan Sun Bear in North-Eastern States of India View project All content following this page was uploaded by Janmejay Sethy on 24 January 2017. The user has requested enhancement of the downloaded file. short communication JANMEJAY SETHY1*, SUSHANTO GOUDA2 AND NETRAPAL SINGH CHAUHAN2 Acknowledgements We are thankful to the Ocean Park Foundation, Photographic evidence of Hongkong, for the financial support in carrying out the project. We would like to thank Mr. Liandawla, marbled cat from Dampa Ti- Chief Wildlife Warden of Mizoram and Mr. Lalrin- mawia, Field Director of Dampa Tiger Reserve for ger Reserve, Mizoram, India permitting us to work in the reserve and their help and support. The authors are also thankful to the The marbled cat Pardofelis marmorata is one of the rarest and the least known felid forest staff of Dampa for their help and co-operati- species. It is listed as Near Threatened in the IUCN Red List and in Appendix I of on during the course of the field work. CITES. In India, the species is restricted to eastern Himalayan foothills, especially Arunachal Pradesh. -

Marbled Cat 1 Marbled Cat
Marbled Cat 1 Marbled Cat Marbled Cat[1] Conservation status [2] Vulnerable (IUCN 3.1) Scientific classification Kingdom: Animalia Phylum: Chordata Class: Mammalia Order: Carnivora Family: Felidae Subfamily: Felinae Genus: Pardofelis Severtzov, 1858 Species: P. marmorata Binomial name Pardofelis marmorata (Martin, 1837) Subspecies • P. m. charltoni • P. m. marmorata Marbled Cat 2 Marbled cat range The Marbled Cat (Pardofelis marmorata) is a small wild cat of South and Southeast Asia. Since 2002 it has been listed as vulnerable by IUCN as it occurs at low densities, and its total effective population size is suspected to be fewer than 10,000 mature individuals, with no single population numbering more than 1,000.[2] The species was once considered to belong to the pantherine lineage of "big cats".[3] Genetic analysis has shown that it is closely related with the Asian Golden Cat and the Bay Cat, all of which diverged from the other felids about 9.4 million years ago.[4] Characteristics The marbled cat is similar in size to a domestic cat, with a more thickly furred tail (which may be longer than the body), showing adaptation to its arboreal life-style, where the tail is used as a counterbalance. Marbled cats range from 45 to 62 centimetres (18 to 24 in) in head-body length, with a 35 to 55 centimetres (14 to 22 in) tail. Recorded weights vary between 2 and 5 kilograms (4.4 and 11 lb).[5] The fur is blotched and banded like a marble, usually compared to the markings of the much larger Indochinese Clouded Leopards or Diard's Clouded Leopards. -

Flat Headed Cat Andean Mountain Cat Discover the World's 33 Small
Meet the Small Cats Discover the world’s 33 small cat species, found on 5 of the globe’s 7 continents. AMERICAS Weight Diet AFRICA Weight Diet 4kg; 8 lbs Andean Mountain Cat African Golden Cat 6-16 kg; 13-35 lbs Leopardus jacobita (single male) Caracal aurata Bobcat 4-18 kg; 9-39 lbs African Wildcat 2-7 kg; 4-15 lbs Lynx rufus Felis lybica Canadian Lynx 5-17 kg; 11-37 lbs Black Footed Cat 1-2 kg; 2-4 lbs Lynx canadensis Felis nigripes Georoys' Cat 3-7 kg; 7-15 lbs Caracal 7-26 kg; 16-57 lbs Leopardus georoyi Caracal caracal Güiña 2-3 kg; 4-6 lbs Sand Cat 2-3 kg; 4-6 lbs Leopardus guigna Felis margarita Jaguarundi 4-7 kg; 9-15 lbs Serval 6-18 kg; 13-39 lbs Herpailurus yagouaroundi Leptailurus serval Margay 3-4 kg; 7-9 lbs Leopardus wiedii EUROPE Weight Diet Ocelot 7-18 kg; 16-39 lbs Leopardus pardalis Eurasian Lynx 13-29 kg; 29-64 lbs Lynx lynx Oncilla 2-3 kg; 4-6 lbs Leopardus tigrinus European Wildcat 2-7 kg; 4-15 lbs Felis silvestris Pampas Cat 2-3 kg; 4-6 lbs Leopardus colocola Iberian Lynx 9-15 kg; 20-33 lbs Lynx pardinus Southern Tigrina 1-3 kg; 2-6 lbs Leopardus guttulus ASIA Weight Diet Weight Diet Asian Golden Cat 9-15 kg; 20-33 lbs Leopard Cat 1-7 kg; 2-15 lbs Catopuma temminckii Prionailurus bengalensis 2 kg; 4 lbs Bornean Bay Cat Marbled Cat 3-5 kg; 7-11 lbs Pardofelis badia (emaciated female) Pardofelis marmorata Chinese Mountain Cat 7-9 kg; 16-19 lbs Pallas's Cat 3-5 kg; 7-11 lbs Felis bieti Otocolobus manul Fishing Cat 6-16 kg; 14-35 lbs Rusty-Spotted Cat 1-2 kg; 2-4 lbs Prionailurus viverrinus Prionailurus rubiginosus Flat