Atlanta Orchid Society Newsletter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Genus Brassavola, (L.) R.Br
The Genus Brassavola, (L.) R.Br. in W.T.Aiton, Hortus Kew. 5: 216 (1813) Type: Brassavola [B.] cucullata [bra-SAH-vo-la kyoo-kyoo-LAH-ta] There are 28 species (OrchidWiz [update Dec 2017]) that are epiphytes and sometimes lithophytes at elevations of from sea level to 3300 ft (1000 m) from Mexico, southern Caribbean islands to northern Argentina in moist or wet montane forests, mangroves, rocky crevices and cliff faces. They are most fragrant at night and many with a citrus smell. The genus is characterized by very small pencil-like pseudobulbs, often forming large clumps; a single, fleshy, apical, sub-terete leaf and the inflorescence produced form the apex of the pseudobulb. The inflorescence carries from a single to a few large flowers. The floral characteristics are elongate narrow similar sepals and petals, the base of the lip usually tightly rolled around at least a portion of the column which carries 12, sometimes eight unequal pollina with prominent opaque caudicles. The flowers usually occur, as a rule, in spring, summer and fall. The flowers are generally yellow to greenish white with a mostly white lip. It is not unusual for dark spots, usually purple, to be in the region where the sepals, petals, and lip join the stem (claw). This spotting is a dominant generic trait in Brassavola nodose. They are easily cultivated under intermediate conditions. Although this is a relatively small genus (28 species), the species show an unusually close relationship with one another in their floral patterns, coloration, and column structure making identification difficult, key to know where the plants were collected. -

New Cattleya Orchid Hybrid(1)
GUILHERME AUGUSTO CITO ALVES CORREIO et. al 145 CULTIVAR DESCRIPTION New Cattleya orchid hybrid(1) GUILHERME AUGUSTO CITO ALVES(2), RODRIGO THIBES HOSHINO(2), DOUGLAS JUNIOR BERTONCELLI(2), RONAN CARLOS COLOMBO(2), VANESSA STEGANI(3), RICARDO TADEU DE FARIA(2) ABSTRACT The hybrid, obtained by back crossing between (Cattleya labiata x Cattleya forbesii) x Cattleya labiata is a vigorous plant, bi- or unifoliate, features slender and cylindrical pseudobulbs and leathery dark-greenish leaves, with oblanceolate shape format of blunt tips with the first flowering four years after sowing. In Londrina, flowering occurred twice a year, between the months of April and May and October and November with 2-4 flowers per pseudobulb and durability ranging from 15 to 20 days. The flowers of the new hybrid were purple with a labellum with a yellow center and purple stripes. Keywords: breeding, floriculture, Orchidaceae, selection. RESUMO Novo híbrido de orquídea Cattleya O híbrido, obtido do retrocruzamento entre (Cattleya labiata x Cattleya forbesii) x Cattleya labiata é uma planta vigorosa, bi ou unifoliada, apresenta pseudobulbos delgados e cilíndricos e folhas coriáceas com tonalidade verde escuro, com formato oblanceolado, de pontas obtusas, apresentando o primeiro florescimento após quatros anos da semeadura. O florescimento em Londrina ocorreu duas vezes no ano, entre os meses de abril e maio e outubro e novembro com 2-4 flores por pseudobulbo e durabilidade entre 15 a 20 dias. As flores do novo híbrido possuem coloração lilás e labelo com um centro amarelo e listras roxas. Palavras-chave: floricultura, melhoramento, Orchidaceae, seleção. 1. INTRODUCTION allows the introduction of relevant genes such as shape, durability, color, and others into hybrids of orchids The Orchidaceae family has about 26,500 species, (WITHNER, 1988) distributed across all continents (KEW, 2011). -

Epidendrum Secundum (Orchidaceae)
Plant Biology ISSN 1435-8603 RESEARCH PAPER Reproductive biology and pollination mechanisms of Epidendrum secundum (Orchidaceae). Floral variation: a consequence of natural hybridization? E. R. Pansarin & M. C. E. Amaral Departamento de Botaˆ nica, Instituto de Biologia, Universidade Estadual de Campinas, Sa˜ o Paulo, Brazil Keywords ABSTRACT Epidendroideae; Epidendrum; Laeliinae; Orchidaceae; pollination; reproductive biology. The phenology, flower morphology, pollination mechanism and reproductive biology of Epidendrum secundum were studied in a semi-deciduous forest at Correspondence the Serra do Japi (SJ), and in the Atlantic rain forest of Picinguaba, both E. R. Pansarin, Departamento de Biologia natural reserves in the State of Sa˜o Paulo, southeastern Brazil. E. secundum Aplicada, Universidade Estadual Paulista, flowers all year round, with a flowering peak between September and FCAV, 14884-900, Jaboticabal, SP, Brazil. January. This species is either a lithophytic or terrestrial herb in the SJ, E-mail: [email protected] whereas, in Picinguaba, it grows mainly in disturbed areas along roadsides. E. secundum is pollinated by several species of diurnal Lepidoptera at both Editor study sites. In Picinguaba, where E. secundum is sympatric with E. fulgens M. Ayasse and both share the same pollinators, pollen transference between these two species was recorded. E. secundum is self-compatible but pollinator-depen- Received: 25 March 2007; Accepted: 22 May dent. It is inter-compatible with E. fulgens, producing fertile seeds. In con- 2007 trast to the population of the SJ, in the Picinguaba region, floral morphology is quite variable among plants and some individuals present doi:10.1111/j.1438-8677.2007.00025.x flowers with characteristics in-between both sympatric species, suggesting that natural hybridization occasionally occurs. -

Encyclia Fimbriata (Orchidaceae: Laeliinae), a New Large-Flowered Species from Bahia, Brazil
Phytotaxa 40: 26–40 (2012) ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ Article PHYTOTAXA Copyright © 2012 Magnolia Press ISSN 1179-3163 (online edition) Encyclia fimbriata (Orchidaceae: Laeliinae), a new large-flowered species from Bahia, Brazil CLÁUDIA A. BASTOS1, CÁSSIO VAN DEN BERG1 & THIAGO E.C. MENEGUZZO2 1Universidade Estadual de Feira de Santana, Programa de Pós-graduação em Botânica. Av. Transnordestina, s.n., 44036-900, Feira de Santana, Bahia, Brazil; email: [email protected] 2 Instituto de Pesquisas Jardim Botânico do Rio de Janeiro, Rua Pacheco Leão, 915, Jardim Botânico, 22460-030, Rio de Janeiro, Rio de Janeiro, Brazil Abstract A new orchid species from Brazil, Encyclia fimbriata, is described and illustrated. It is known only from montane rain forest in southern Bahia. Flower morphology, especially the fringed midlobe of the labellum, crested callus and teeth of the clinandrium, distinguish it from any other species of the genus. Key words: Epiphytes, flora of Bahia, Neotropics Resumo Uma nova espécie de orquídea do Brasil, Encyclia fimbriata, é descrita e ilustrada. Esta é somente conhecida de floresta montana do sul da Bahia. A morfologia da flor, especialmente o lobo medial do labelo fimbriado, o calo sinuoso e os dentes do clinândrio, a diferenciam de todas as demais espécies do gênero. Palavras-chave: Epífitas, flora da Bahia, Neotrópico Introduction Encyclia is the second largest genus in the Neotropical subtribe Laeliinae, being surpassed only by Epidendrum (Dressler 1993, Pridgeon et al. 2003, Chase et al. 2004). Many Encyclia species have ornamental value due to their large and showy flowers. There are about 120 species occuring from Florida, Mexico and West Indies to Brazil and northern Argentina (Withner 1998, 2000, van den Berg & Carnevali F.-C. -

CITES Orchid Checklist Volumes 1, 2 & 3 Combined
CITES Orchid Checklist Online Version Volumes 1, 2 & 3 Combined (three volumes merged together as pdf files) Available at http://www.rbgkew.org.uk/data/cites.html Important: Please read the Introduction before reading this Part Introduction - OrchidIntro.pdf Part I : All names in current use - OrchidPartI.pdf (this file) Part II: Accepted names in current use - OrchidPartII.pdf Part III: Country Checklist - OrchidPartIII.pdf For the genera: Aerangis, Angraecum, Ascocentrum, Bletilla, Brassavola, Calanthe, Catasetum, Cattleya, Constantia, Cymbidium, Cypripedium, Dendrobium (selected sections only), Disa, Dracula, Encyclia, Laelia, Miltonia, Miltonioides, Miltoniopsis, Paphiopedilum, Paraphalaenopsis, Phalaenopsis, Phragmipedium, Pleione, Renanthera, Renantherella, Rhynchostylis, Rossioglossum, Sophronitella, Sophronitis Vanda and Vandopsis Compiled by: Jacqueline A Roberts, Lee R Allman, Sharon Anuku, Clive R Beale, Johanna C Benseler, Joanne Burdon, Richard W Butter, Kevin R Crook, Paul Mathew, H Noel McGough, Andrew Newman & Daniela C Zappi Assisted by a selected international panel of orchid experts Royal Botanic Gardens, Kew Copyright 2002 The Trustees of The Royal Botanic Gardens Kew CITES Secretariat Printed volumes: Volume 1 first published in 1995 - Volume 1: ISBN 0 947643 87 7 Volume 2 first published in 1997 - Volume 2: ISBN 1 900347 34 2 Volume 3 first published in 2001 - Volume 3: ISBN 1 84246 033 1 General editor of series: Jacqueline A Roberts 2 Part I: ORCHIDACEAE BINOMIALS IN CURRENT USAGE Ordered alphabetically on All -

Gainesville Orchid Society Newsletter
GainesvilleGainesville OrchidOrchid SocietySociety Newsletter Newsletter EDITORS: MALLORIE AN D J U N E 2 0 1 1 MATTHEW GAUGHRAN Elected Officers: President - Georgia Shemitz 386-454-2147(hm) President’s Message– June 2011 352-283-2022(cell) Gainesville Orchid Society Show Vice-President - N/A The time has come to plan our annual show. It will be held at Kanahapa Botanical Gardens on October Secretary – Linda 15-16. I find that helping with the show is a great way to socialize with GOS members, interact with White vendors, and with folks from other societies. I am inspired by all of the beautiful orchids. I always see 352-284-3849 Treasurer – Susannah something new and learn a thing or two along the way. I hope that all of you will take part. Here is the Link schedule of events: 352-514-1911 Enter Your Orchids---Wednesday-Thursday, October 12-13 Permanent Commit- Enter your best blooming orchids. tees: Set-up--- Friday, October 14, 8am-until we’re done Beginners‟ Classes - Johanna Willink 352-372- Set up is a lot of hard work, but I think it is the most fun part of the entire show. We start with two 6624 empty rooms and by the end of the day they are transformed into an orchid paradise. Tasks during set- Greenhouse Tours - up include helping to set up the GOS display, unloading and distributing background plants, helping the Roy Cline vendors and societies unload. There will be coffee in the morning and lunch is provided. Come for the 352-336-8523 whole day, an hour or two, or just stop by to see what is going on. -
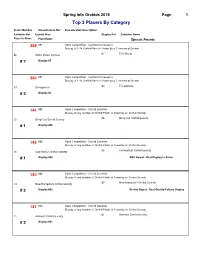
COS 2019 Show Ribbon Awards by Class
Spring Into Orchids 2019 Page 1 Top 3 Placers By Category Ticket Number Classification Ref Classification Description Exhibitor Ref Exhibit Area Display Ref Exhibitor Name Place In Class Plant Name Special Awards 359 001 Open Competition - Commercial Growers Display of 1-24 Orchid Plants in flower by a Commercial Grower 07 Eric Wang 06 White Plains Orchids # 2 Display #7 360 001 Open Competition - Commercial Growers Display of 1-24 Orchid Plants in flower by a Commercial Grower 03 Ecuagenera 37 Ecuagenera # 3 Display #3 144 003 Open Competition - Orchid Societies Display of any number of Orchid Plants in flower by an Orchid Society 06 Deep Cut Orchid Society 15 Deep Cut Orchid Society # 1 Display #06 145 003 Open Competition - Orchid Societies Display of any number of Orchid Plants in flower by an Orchid Society 08 Connecticut Orchid Society 16 Connecticut Orchid Society # 1 Display #08 AOS Award - Best Display in Show 140 003 Open Competition - Orchid Societies Display of any number of Orchid Plants in flower by an Orchid Society 05 New Hampshire Orchid Society 14 New Hampshire Orchid Society # 2 Display #05 Orchid Digest - Best Quality/Culture Display 141 003 Open Competition - Orchid Societies Display of any number of Orchid Plants in flower by an Orchid Society 01 Amherst Orchid Society 11 Amherst Orchid Society # 2 Display #01 Spring Into Orchids 2019 Page 2 Top 3 Placers By Category Ticket Number Classification Ref Classification Description Exhibitor Ref Exhibit Area Display Ref Exhibitor Name Place In Class Plant Name Special Awards 143 003 Open Competition - Orchid Societies Display of any number of Orchid Plants in flower by an Orchid Society 04 Cape & Islands Orchid Society 13 Cape & Islands Orchid Society # 2 Display #04 100 011 Cattleya Alliance(Laeliinae) Encyclia species 05 Chuck & Sue Andersen 10 New Hampshire Orchid Society # 1 Encyclia vitellina 67 011 Cattleya Alliance(Laeliinae) Encyclia species 07 Eric Wang 06 White Plains Orchids # 2 Enc. -

4 CITES Listed Species
Dutch Caribbean Species of High Conservation Value 4 CITES listed species 4.1 Notes on CITES Species CITES (the Convention on International Trade in Endangered Species of Wild Fauna and Flora) is an international agreement between governments. Its aim is to ensure that international trade in specimens of wild animals and plants does not threaten their survival. In the CITES legislation organisms are classified in 3 categories depending on how endangered they are thought to be, or the degree of protection they are thought to need (CITES-Website 2012); ! Appendix I includes species threatened with extinction. Trade in specimens of these species is permitted only in exceptional circumstances. Import and export or re-export permits are required, issued by the Management Authority of the State of Import, Export or Re-Export. ! Appendix II includes species where trade is allowed but restricted. An export or re-export certificate is required, but no import permit is needed unless required by national law. ! Appendix III are species in which trade is allowed but monitored. An export permit must be issued by the Management Authority of the State that included the species in Appendix III. 4.2 Summary data CITES I CITES II CITES I CITES II CITES Dutch Caribbean 15 189 Saba 16 153 Marine 12 1120 Marine 5 115 Terrestrial 3 68 Terrestrial 1 36 Aruba 7 124 St Eustatius 160 159 Marine 4 102 Marine 5 115 Terrestrial 3 21 Terrestrial 1 43 Bonaire 17 153 St Maarten 16 152 Marine 5 115 Marine 5 115 Terrestrial 2 31 Terrestrial 1 33 Curaçao 17 158 Marine 6 115 Terrestrial 1 36 CITES listed species 31 November 2012 Dutch Caribbean Species of High Conservation Value 4.3 CITES I 4.3.1 Marine CITES Appendix I species Common Group Name English Name Scientific name Notes Aruba Bonaire Curaçao Saba Eustatius St. -

Species Data Sheet Cattleya Milleri (Blumensch
Species Data Sheet Cattleya milleri (Blumensch. ex Pabst) Van den Berg, Neodiversity 3: 9 (2008) [KAT-lee-a mil-LAR-ee] Recently ‘found’, 1960, in Brazil as a miniature sized, warm to cool growing "rupicolous" lithophyte on iron ore outcroppings in Minas Gerais or on the base of Vellozia shrubs at elevations of 2500 to 4300 feet (800 to 1300 m). The clusterd red pseudobulbs are up to 2.5” (6 cm) high, widest at the base, and bear a stiff, somewhat narrow 4.0” (10 cm) ovate-oblong, keeled, rigid leaf with a sharp tip. The leaf is borne at an angle to the pseudobulb, not in line with it, and the foliage of most plants has a deep maroon flush, particularly on the backs of the leaves. The blood- or range-red starry flowers approach 2.0” (5.0 cm) in diameter, blooming in late spring and early summer (May through July) on a erect, 12 to 18" [30 to 50 cm] long, several to many flowered (approximately 6 to 12 flowers at the same time), Cattleya milleri racemose inflorescence with successive opening flowers, held well ‘Phyllis’ AM/AOS above the leaves. The lip is usually yellow with cinnabar-colored May 2010, NS 5.0 x 5.0 cm veining and varying width picotee. There are two color types, one group has narrower sepals and petals, slightly larger flowers and an orange-red color. The other group has slightly smaller flowers with wider sepals and petals and their color is a more blood-red. Synonyms: Laelia milleri Varieties / forms: None. -

Epidendrum L. (Orchidaceae, Epidendroideae) No Parque Nacional Da Chapada Dos Veadeiros, Estado De Goiás, Brasil
Artigo Hoehnea 47: e202020, 9 fig., 2020 http://dx.doi.org/10.1590/2236-8906-20/2020 Epidendrum L. (Orchidaceae, Epidendroideae) no Parque Nacional da Chapada dos Veadeiros, Estado de Goiás, Brasil Igor Soares dos Santos1,2 & Marcos José da Silva1 Recebido: 20.03.2020; aceito: 05.08.2020 Como citar: Santos, I.S. & Silva, M.J. 2020. Epidendrum L. (Orchidaceae, Epidendroideae) no Parque Nacional da Chapada dos Veadeiros, Estado de Goiás, Brasil. Hoehnea 47: e202020. http://dx.doi.org/10.1590/2236-8906-20/2020. RESUMO – (Epidendrum L. (Orchidaceae, Epidendroideae) no Parque Nacional da Chapada dos Veadeiros, Estado de Goiás, Brasil). Epidendrum L. é um dos maiores gêneros de Orchidaceae Juss. com 2.400 espécies neotropicais, 121 das quais presentes no Brasil, sendo 69 delas endêmicas. A taxonomia das espécies de Epidendrum na região Centro-Oeste é escassamente conhecida e vinculada a estudos florísticos sobre Orchidaceae. É apresentado o tratamento taxonômico às espécies de Epidendrum ocorrentes no Parque Nacional da Chapada dos Veadeiros, uma das Áreas de Preservação Permanentes mais importantes do Brasil. Foram encontradas seis espécies: E. avicula Lindl., E. campacci Hágsater & L. Sánchez, E. dendrobioides Thunb., E. nocturnum Jacq., E. rothii A.D. Hawkes e E. secundum Jacq., crescendo como epífitas, terrícolas e rupícolas em distintas fitofisionomias. As espécies seguem descritas e ilustradas, comentadas quanto a distribuição geográfica, relações morfológicas, fenologia, bem como contrastadas por meio de uma chave dicotômica e alocadas em grupos informais reconhecidos para o gênero. Palavras-chave: Cerrado, diversidade, flora, orquídeas, Taxonomia ABSTRACT – (Epidendrum L. (Orchidaceae, Epidendroideae) in the Parque Nacional da Chapada dos Veadeiros, Goiás State, Brazil). -

Classification Index - Printed on 10/13/2014 Page 1
Classification Index - Printed On 10/13/2014 Page 1 Reference Classification Description 001 Open Competition - Commercial Growers Display of 1-24 Orchid Plants in flower by a Commercial Grower 002 Open Competition - Commercial Grower Display of 25 or more Orchid Plants in flower by a Commercial Grower 003 Open Competition - Orchid Societies Display of any number of Orchid Plants in flower by an Orchid Society 004 Open Competition - Non-Commercial Growers Display of any number of Orchid Plants in flower by a Non-Commercial grower but not an Orchid Society 005 Open Competition - Educational Educational exhibits, any size 006 Open Competition - Artwork Display of artwork of any type, any size 007 Open Competition - Cut Flower & Floral Arrangement Display of cut flowers and floral arrangements containing orchids, any size 008 Blank Class 009 Blank Class 010 Cattleya Alliance (Laeliinae) Epidendrum species 011 Cattleya Alliance(Laeliinae) Encyclia species 012 Cattleya Alliance (Laeliinae) Epidendrum and Encyclia hybrids and intergeneric hybrids with any genera INCLUDING Cattleya 013 Cattleya Alliance (Laeliinae) Brassovola and Rhyncholaelia species, hybrids, and intergeneric hybrids other than above but EXCLUDING Cattleya, e.g.Bl, Rl 014 Cattleya Alliance (Laeliinae) Laelia species, hybrids, and intergeneric hybrids other than above but EXCLUDING Cattleya Classification Index - Printed On 10/13/2014 Page 2 Reference Classification Description 015 Cattleya Alliance (Laeliinae) Sophronitis species, hybrids, and intergeneric hybrids other than above but EXCLUDING Cattleya 016 Cattleya Alliance (Laeliinae) Schomburgkia species, hybrids, and intergeneric hybrids other than above INCLUDING Cattleya, ex. Schombocattleya 017 Cattleya Alliance (Laeliinae) Broughtonia species, hybrids, and intergeneric hybrids other than above INCLUDING Cattleya, ex. -

Caribbean Area Endangered and Threatened Species List Virgin Islands
CARIBBEAN AREA ENDANGERED AND THREATENED SPECIES LIST VIRGIN ISLANDS Protection Scientific name Common name Range Level ANIMALS Birds Sterna dougallii ** Roseate tern Migrant F Pelecanus occidentalis * Brown pelican Resident F Falco peregrinus * Peregrine falcon Winter migrant F Puffinus lherminieri Audobon shearwater Migrant VIL Sterna antillarum Least tern Migrant VIL Oxyura jamaicensis Ruddy duck Peripheral resident VIL Anas bahamensis Bahama duck Resident VIL Anthracothorax dominicus Antillean mango Resident VIL Aratinga pertinax Brown-throated Parakeet Resident VIL Ardea herodias Great blue heron Resident VIL Casmerodius albus Great (common) egret Resident VIL Catoptrophorus semipalmatus Willet Resident VIL Charadrius alexandrinus Snowy Plover Resident VIL Chordeiles gundlachii West Indian nighthawk Resident VIL Columba leucocephala White-crowned Pigeon Resident VIL Egretta thula Snowy egret Resident VIL Fulica caribaea Caribbean Coot Resident VIL Ixobrychus exilis Least bittern Resident VIL Myiarchus stolidus Stolid Flycatcher Resident VIL Nycticorax nycticorax Black-crowned night-heron Resident VIL Otus nudipes newtoni Virgin Islands screech owl Resident VIL Phaethon lepturus White-tailed tropicbird Resident VIL Podiceps dominicus Least grebe Resident VIL Rallus longirostris Clapper Rail Resident VIL Geotrygon mystacea Bridled Quail Dove Resident VIL Mammals Brachyphylla cavernarum Cave bat Resident VIL Noctilio leporinus Fisherman bat Resident VIL Stenoderma rufum Red fruit bat Resident VIL ReptiIia Ameiva polops * St. Croix ground lizard Resident F Chelonia mydas ** Green turtle Resident F Dermochelys coriacea * Leatherback sea turtle Migrant F Epicrates monensis granti * Virgin Islands tree boa Resident F Eretmochelys imbricata ** Hawksbill sea turtle Resident F Mabuya mabouia Slipperyback skink Resident VIL 9/11/02 2002endangered-threatened.xls CARIBBEAN AREA ENDANGERED AND THREATENED SPECIES LIST VIRGIN ISLANDS Protection Scientific name Common name Range Level PLANTS Cacti Mammilaria nivosa Wooly nipple St.