CMS Snapshot April 8-15, 2021 (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DOE Family Update: April 8, 2021
DOE Family Update April 8, 2021 Contents • All families: Last day to opt-in to in-person learning extended to Friday, April 9 • Families applying to Gifted & Talented kindergarten programs: Application deadline is Friday, April 9 • Families applying to pre-K: Application deadline extended to Monday, April 19 • Families with children in grades 3–8: New York State Exam update Important Deadlines Spring Opt-in Deadline Extended to Friday, April 9 If your child is currently learning remotely every day, they have one final opportunity to opt in to learning in person in the school building at least part of the week. The deadline to submit your request to transition to blended learning for the rest of this school year has been extended to Friday, April 9. How to opt-in to blended learning: • Online: visit the Learning Preference Survey at nycenet.edu/surveys/learningpreference to select blended learning for your child. • By phone: Call 311 to submit your learning preference change. G&T Kindergarten Application Deadline is Friday, April 9 The application for kindergarten Gifted & Talented (G&T) programs is now open, and the deadline to apply is April 9. Interested families with children born in 2016 can apply one of three ways: • Online at MySchools.nyc • Through a Family Welcome Center—visit schools.nyc.gov/fwc to learn more. • By phone at 718-935-2009—you can also call us with any questions. Pre-K Application Deadline Extended to Monday, April 19 Now you have more time to explore your child’s pre-K options! The pre-K application deadline for children born in 2017 has been extended to April 19. -

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

2019-2022 Calendar of Major Jewish Holidays
2019-2022 CALENDAR OF MAJOR JEWISH HOLIDAYS Please note: Jewish students may not be able to participate in school activities that take place on the days marked with an *. 2019 2020 2021 2022 PURIM Celebrates the defeat of the plot to destroy March 21 March 10 February 26 March 17 the Jews of Persia. PASSOVER Deliverance of the Jewish people from Egypt. The first *Eve. of April 19 *Eve. of April 8 *Eve. of March 27 *Eve of April 15 and last two days are observed as full holidays. There are *April 20 *April 9 *March 28 *April 16 dietary restrictions against leavened products (such as *April 21 *April 10 *March 29 *April17 bread, pastries, pasta, certain legumes and more) during *April 26 *April 15 *April 3 *April 21 all eight days of the holiday. *April 27 *April 16 *April 4 *April 22 SHAVUOT *Eve. of June 8 *Eve. of May 28 *Eve. of May 16 *Eve of June 3 Feast of Weeks, marks the giving of the Law (Torah) *June 9 *May 29 *May 17 *June 4 at Mt. Sinai. (Often linked with the Confirmation *June 10 *May 30 *May 18 *June 5 of teenagers.) ROSH HASHANAH *Eve. of Sept. 29 *Eve. of Sept. 18 *Eve. of Sept. 6 *Eve of Sept 25 The Jewish New Year; start of the Ten Days of Penitence. *Sept. 30 *Sept. 19 *Sept. 7 *Sept. 26 The first two days are observed as full holidays. *Oct. 1 *Sept. 20 *Sept. 8 *Sept. 27 YOM KIPPUR Day of Atonement; the most solemn day *Eve. -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -
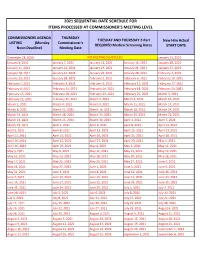
2021 Sequential Date List
2021 SEQUENTIAL DATE SCHEDULE FOR ITEMS PROCESSED AT COMMISSIONER'S MEETING LEVEL COMMISSIONERS AGENDA THURSDAY TUESDAY AND THURSDAY 2-Part New Hire Actual LISTING (Monday Commissioner's REQUIRED Medical Screening Dates START DATE Noon Deadline) Meeting Date December 28, 2020 NO MEETING SCHEDULED January 13, 2021 January 4, 2021 January 7, 2021 January 12, 2021 January 14, 2021 January 20, 2021 January 11, 2021 January 14, 2021 January 19, 2021 January 21, 2021 January 27, 2021 January 18, 2021 January 21, 2021 January 26, 2021 January 28, 2021 February 3, 2021 January 25, 2021 January 28, 2021 February 2, 2021 February 4, 2021 February 10, 2021 February 1, 2021 February 4, 2021 February 9, 2021 February 11, 2021 February 17, 2021 February 8, 2021 February 11, 2021 February 16, 2021 February 18, 2021 February 24, 2021 February 15, 2021 February 18, 2021 February 23, 2021 February 25, 2021 March 3, 2021 February 22, 2021 February 25, 2021 March 2, 2021 March 4, 2021 March 10, 2021 March 1, 2021 March 4, 2021 March 9, 2021 March 11, 2021 March 17, 2021 March 8, 2021 March 11, 2021 March 16, 2021 March 18, 2021 March 24, 2021 March 15, 2021 March 18, 2021 March 23, 2021 March 25, 2021 March 31, 2021 March 22, 2021 March 25, 2021 March 30, 2021 April 1, 2021 April 7, 2021 March 29, 2021 April 1, 2021 April 6, 2021 April 8, 2021 April 14, 2021 April 5, 2021 April 8, 2021 April 13, 2021 April 15, 2021 April 21, 2021 April 12, 2021 April 15, 2021 April 20, 2021 April 22, 2021 April 28, 2021 April 19, 2021 April 22, 2021 April 27, 2021 April -

Date of Close Contact Exposure
Date of Close Contact Exposure 7 days 10 days 14 days Monday, November 16, 2020 Tuesday, November 24, 2020 Friday, November 27, 2020 Tuesday, December 1, 2020 Tuesday, November 17, 2020 Wednesday, November 25, 2020 Saturday, November 28, 2020 Wednesday, December 2, 2020 Wednesday, November 18, 2020 Thursday, November 26, 2020 Sunday, November 29, 2020 Thursday, December 3, 2020 Thursday, November 19, 2020 Friday, November 27, 2020 Monday, November 30, 2020 Friday, December 4, 2020 Friday, November 20, 2020 Saturday, November 28, 2020 Tuesday, December 1, 2020 Saturday, December 5, 2020 Saturday, November 21, 2020 Sunday, November 29, 2020 Wednesday, December 2, 2020 Sunday, December 6, 2020 Sunday, November 22, 2020 Monday, November 30, 2020 Thursday, December 3, 2020 Monday, December 7, 2020 Monday, November 23, 2020 Tuesday, December 1, 2020 Friday, December 4, 2020 Tuesday, December 8, 2020 Tuesday, November 24, 2020 Wednesday, December 2, 2020 Saturday, December 5, 2020 Wednesday, December 9, 2020 Wednesday, November 25, 2020 Thursday, December 3, 2020 Sunday, December 6, 2020 Thursday, December 10, 2020 Thursday, November 26, 2020 Friday, December 4, 2020 Monday, December 7, 2020 Friday, December 11, 2020 Friday, November 27, 2020 Saturday, December 5, 2020 Tuesday, December 8, 2020 Saturday, December 12, 2020 Saturday, November 28, 2020 Sunday, December 6, 2020 Wednesday, December 9, 2020 Sunday, December 13, 2020 Sunday, November 29, 2020 Monday, December 7, 2020 Thursday, December 10, 2020 Monday, December 14, 2020 Monday, November -

Dates for Student-Run Credit Unions 15-16 Northville Schools Northern Schools Novi & Livonia Schools
Dates for Student-Run Credit Unions 15-16 Northville Schools Northern Schools Novi & Livonia Schools Plymouth-Canton Elementary Schools: Bentley Bird Dodson Eriksson Credit Union Credit Union Credit Union Credit Union Tuesdays: Thursdays: October 6 Wednesdays: Tuesdays: October 13 October 20 October 7 October 20 October 27 November 10 October 28 November 10 November 17 November 24 November 18 December 1 December 8 December 8 December 16 December 15 January 5 January 12 January 5 January 6 January 19 January 26 January 19 January 27 February 2 February 9 February 2 February 10 February 23 March 1 February 23 March 2 March 8 March 15 March 8 March 23 March 22 April 5 March 22 April 13 April 12 April 5 May 4 April 19 April 26 April 19 May 25 May 3 May 10 May 17 May 3 Farrand Field Gallimore Hoben Credit Union Credit Union Credit Union Credit Union Wednesdays: Tuesdays: Thursdays: Thursdays: October 21 October 20 October 8 October 15 November 11 November 17 October 22 November 12 December 9 December 1 November 12 December 10 January 6 December 15 December 3 January 14 February 3 January 12 January 7 February 11 February 24 January 26 March 10 March 16 February 4 February 9 April 14 April 6 February 25 February 23 May 12 May 4 March 8 March 10 March 22 April 7 April 12 April 28 April 26 May 12 May 10 June 2 Hulsing Isbister Credit Miller Smith Credit Union Union Credit Union Credit Union Thursdays: Wednesdays: October 1 Thursdays: Thursdays: September 30 October 15 October 8 October 1 October 14 November 19 November 5 October 22 October 28 -

April 8, 2021 As of Yesterday's Report, Greenwood County Had 12
April 8, 2021 As of yesterday's report, Greenwood County had 12 additional confirmed cases in the county for a total of 7,158 cases confirmed since 21 March 2020. Remember this is only those which have been confirmed and is for those who were tested a minimum of two days ago. This means there are others who have been tested and are in quarantine awaiting results. Additionally, an individual can be asymptomatic, (contagious but shows no symptoms), and may choose not to be tested. The first 7,002 cases in Greenwood County were reported over 15 days ago, so there are 7,002 individuals who should have recovered from the virus. Additionally, Greenwood County has had 150 confirmed deaths due to the virus. This brings Greenwood County's remaining estimated total of active confirmed cases to 16. DHEC does not track recovered individuals by county, so this number is an estimate based on the following: Most individuals recover from the virus within this time frame. From 23 March until 6 April (2 weeks), Greenwood has had 141 confirmed cases out of a population of 70,811. This equates to an incident rate of 199.1 per 100,000 individuals which is rated as medium by SCDHEC. (Low = 50 or less; Medium = 51 - 200; High = 201 or more) SCDHEC reported an additional 324 new confirmed cases in the state for a total of 468,939. Also, they reported 6 new confirmed deaths in the state for a total of 8,118. Please click this link to see the deaths by county. -

April 8, 2021
April 8, 2021 Greetings, In this newsletter you will find the latest news, announcements and event details that I want to share with you, including COVID-19 testing and mitigation efforts, library reopening information, paid summer internships for youth and young adults, and much more. As always, please do not hesitate to contact my office with questions, comments or concerns at (602) 262-7441 or [email protected]. Respectfully, Debra Stark Councilwoman City of Phoenix Council District 3 Face Coverings Remain in Effect Within Phoenix City Limits After consideration and review with legal counsel, the City of Phoenix has determined the June 19, 2020, Declaration of the Phoenix City Council requiring face coverings in public for most people within city limits remains in effect. The mandate follows Arizona Department of Public Health Face Covering Guidance and is in accordance with current Centers for Disease Control guidance stating that masks should be worn in addition to staying at least 6 feet apart to prevent the spread of COVID-19. At this time, the Council has decided not to rescind this declaration, joining with other municipalities including Tucson and Pima County in reaffirming the requirement to wear masks. The mask mandate within the City of Phoenix will remain in place until such time as the Council votes to terminate or amend the declaration. For more information on this declaration, visit the PHXNewsroom. FREE COVID-19 Testing The Mobile Testing Van #1 will be offering free rapid and antigen tests in District 3 next week! Thursday, April 15 7:15 a.m. -

2021 Working Day Calendar-5 Day-Alternative Format
2021 Working Days Calendar – 5 day The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 Non-working Day Saturday, January 2, 2021 Non-working Day Sunday, January 3, 2021 Non-working Day Monday, January 4, 2021 938 Tuesday, January 5, 2021 939 Wednesday, January 6, 2021 940 Thursday, January 7, 2021 941 Friday, January 8, 2021 942 Saturday, January 9, 2021 Non-working Day Sunday, January 10, 2021 Non-working Day Monday, January 11, 2021 943 Tuesday, January 12, 2021 944 Wednesday, January 13, 2021 945 Thursday, January 14, 2021 946 Friday, January 15, 2021 947 Saturday, January 16, 2021 Non-working Day Sunday, January 17, 2021 Non-working Day Monday, January 18, 2021 Non-working Day Tuesday, January 19, 2021 948 Wednesday, January 20, 2021 949 Thursday, January 21, 2021 950 Friday, January 22, 2021 951 Saturday, January 23, 2021 Non-working Day Sunday, January 24, 2021 Non-working Day Monday, January 25, 2021 952 Tuesday, January 26, 2021 953 Wednesday, January 27, 2021 954 Thursday, January 28, 2021 955 Friday, January 29, 2021 956 Saturday, January 30, 2021 Non-working Day Sunday, January 31, 2021 Non-working Day Monday, February 1, 2021 957 Tuesday, February 2, 2021 958 Wednesday, February 3,