Standard Format for AOAC Standard Method Performance Requirement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
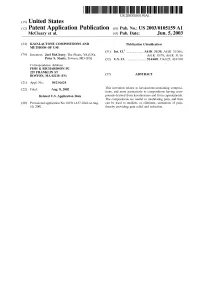
(12) Patent Application Publication (10) Pub. No.: US 2003/0105159 A1 Mccleary Et Al
US 200301 05159A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0105159 A1 McCleary et al. (43) Pub. Date: Jun. 5, 2003 (54) KAVALACTONE COMPOSITIONS AND Publication Classification METHODS OF USE (51) Int. Cl." ....................... A61K 31/35; A61K 31/366; (76) Inventors: Joel McCleary, The Plains, VA (US); A61K 35/78; A61K 31/16 Peter S. Staats, Towson, MD (US) (52) U.S. Cl. ........................... 514/460; 514/625; 424/760 Correspondence Address: FISH & RICHARDSON PC 225 FRANKLIN ST BOSTON, MA 02110 (US) (57) ABSTRACT (21) Appl. No.: 10/214,624 (22) Filed: Aug. 8, 2002 This invention relates tO kavalactone-containing composi tions, and more particularly to compositions having com Related U.S. Application Data pounds derived from kavalactones and from capsaicinoids. The compositions are useful in modulating pain, and thus (60) Provisional application No. 60/311,437, filed on Aug. can be used to mediate, or eliminate, Sensations of pain, 10, 2001. thereby providing pain relief and reduction. US 2003/0105159 A1 Jun. 5, 2003 KAVALACTONE COMPOSITIONS AND METHODS 0006. In one embodiment, the invention relates to an OF USE analgesic topical composition having: (a) a kavalactone; (b) capsaicinoid or Synthetic derivatives thereof; and (c) a CROSS-REFERENCE TO RELATED pharmaceutically acceptable carrier; wherein the weight APPLICATIONS ratio of(a):(b) is from 5000:1 to 1:2 (e.g., 800:1 to 1:1; 500:1 to 5:1). In other aspects, the composition includes an effec 0001. This application claims benefit of U.S. application tive amount of kavalactones, active kavalactones, or capsai Ser. -

Yangonin Blocks Tumor Necrosis Factor-Α–Induced Nuclear Factor-Κb–Dependent Transcription by Inhibiting the Transactivation Potential of the Rela/P65 Subunit
J Pharmacol Sci 118, 447 – 454 (2012) Journal of Pharmacological Sciences © The Japanese Pharmacological Society Full Paper Yangonin Blocks Tumor Necrosis Factor-α–Induced Nuclear Factor-κB–Dependent Transcription by Inhibiting the Transactivation Potential of the RelA/p65 Subunit Juan Ma1,†, He Liang1,†, Hong Ri Jin2, Nguyen Tien Dat3, Shan Yu Zhang1, Ying Zi Jiang1, Ji Xing Nan1, Donghao Li1, Xue Wu1, Jung Joon Lee1,2,*a, and Xuejun Jin1,*b 1Key Laboratory of Natural Resources of Changbai Mountain & Functional Molecules, Yanbian University, Ministry of Education, Yanji Jilin 133002, China 2Center for Molecular Cancer Research, Korea Research Institute of Bioscience and Biotechnology, Ochang, Chungbuk 363-883, Republic of Korea 3Institute of Marine Biochemistry, Vietnam Academy of Science and Technology, 18-Hoang Quoc Viet, Hanoi, Vietnam Received November 13, 2011; Accepted January 23, 2012 Abstract. The nuclear factor-κB (NF-κB) transcription factors control many physiological pro- cesses including inflammation, immunity, and apoptosis. In our search for NF-κB inhibitors from natural resources, we identified yangonin from Piper methysticum as an inhibitor of NF-κB activa- tion. In the present study, we demonstrate that yangonin potently inhibits NF-κB activation through suppression of the transcriptional activity of the RelA/p65 subunit of NF-κB. This compound sig- nificantly inhibited the induced expression of the NF-κB-reporter gene. However, this compound did not interfere with tumor necrosis factor-α (TNF-α)-induced inhibitor of κBα (IκBα) degrada- tion, p65 nuclear translocation, and DNA-binding activity of NF-κB. Further analysis revealed that yangonin inhibited not only the induced NF-κB activation by overexpression of RelA/p65, but also transactivation activity of RelA/p65. -

Herbal Insomnia Medications That Target Gabaergic Systems: a Review of the Psychopharmacological Evidence
Send Orders for Reprints to [email protected] Current Neuropharmacology, 2014, 12, 000-000 1 Herbal Insomnia Medications that Target GABAergic Systems: A Review of the Psychopharmacological Evidence Yuan Shia, Jing-Wen Donga, Jiang-He Zhaob, Li-Na Tanga and Jian-Jun Zhanga,* aState Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, P.R. China; bDepartment of Pharmacology, School of Marine, Shandong University, Weihai, P.R. China Abstract: Insomnia is a common sleep disorder which is prevalent in women and the elderly. Current insomnia drugs mainly target the -aminobutyric acid (GABA) receptor, melatonin receptor, histamine receptor, orexin, and serotonin receptor. GABAA receptor modulators are ordinarily used to manage insomnia, but they are known to affect sleep maintenance, including residual effects, tolerance, and dependence. In an effort to discover new drugs that relieve insomnia symptoms while avoiding side effects, numerous studies focusing on the neurotransmitter GABA and herbal medicines have been conducted. Traditional herbal medicines, such as Piper methysticum and the seed of Zizyphus jujuba Mill var. spinosa, have been widely reported to improve sleep and other mental disorders. These herbal medicines have been applied for many years in folk medicine, and extracts of these medicines have been used to study their pharmacological actions and mechanisms. Although effective and relatively safe, natural plant products have some side effects, such as hepatotoxicity and skin reactions effects of Piper methysticum. In addition, there are insufficient evidences to certify the safety of most traditional herbal medicine. In this review, we provide an overview of the current state of knowledge regarding a variety of natural plant products that are commonly used to treat insomnia to facilitate future studies. -

Herbal Medicines in Pregnancy and Lactation : an Evidence-Based
00 Prelims 1410 10/25/05 2:13 PM Page i Herbal Medicines in Pregnancy and Lactation An Evidence-Based Approach Edward Mills DPh MSc (Oxon) Director, Division of Clinical Epidemiology Canadian College of Naturopathic Medicine North York, Ontario, Canada Jean-Jacques Duguoa MSc (cand.) ND Naturopathic Doctor Toronto Western Hospital Assistant Professor Division of Clinical Epidemiology Canadian College of Naturopathic Medicine North York, Ontario, Canada Dan Perri BScPharm MD MSc Clinical Pharmacology Fellow University of Toronto Toronto, Ontario, Canada Gideon Koren MD FACMT FRCP Director of Motherisk Professor of Medicine, Pediatrics and Pharmacology University of Toronto Toronto, Ontario, Canada With a contribution from Paul Richard Saunders PhD ND DHANP 00 Prelims 1410 10/25/05 2:13 PM Page ii © 2006 Taylor & Francis Medical, an imprint of the Taylor & Francis Group First published in the United Kingdom in 2006 by Taylor & Francis Medical, an imprint of the Taylor & Francis Group, 2 Park Square, Milton Park, Abingdon, Oxon OX14 4RN Tel.: ϩ44 (0)20 7017 6000 Fax.: ϩ44 (0)20 7017 6699 E-mail: [email protected] Website: www.tandf.co.uk/medicine All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or trans- mitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission of the publisher or in accordance with the provisions of the Copyright, Designs and Patents Act 1988 or under the terms of any licence permitting limited copying issued by the Copyright Licensing Agency, 90 Tottenham Court Road, London W1P 0LP. -

Plant-Based Medicines for Anxiety Disorders, Part 2: a Review of Clinical Studies with Supporting Preclinical Evidence
CNS Drugs 2013; 24 (5) Review Article Running Header: Plant-Based Anxiolytic Psychopharmacology Plant-Based Medicines for Anxiety Disorders, Part 2: A Review of Clinical Studies with Supporting Preclinical Evidence Jerome Sarris,1,2 Erica McIntyre3 and David A. Camfield2 1 Department of Psychiatry, Faculty of Medicine, University of Melbourne, Richmond, VIC, Australia 2 The Centre for Human Psychopharmacology, Swinburne University of Technology, Melbourne, VIC, Australia 3 School of Psychology, Charles Sturt University, Wagga Wagga, NSW, Australia Correspondence: Jerome Sarris, Department of Psychiatry and The Melbourne Clinic, University of Melbourne, 2 Salisbury Street, Richmond, VIC 3121, Australia. Email: [email protected], Acknowledgements Dr Jerome Sarris is funded by an Australian National Health & Medical Research Council fellowship (NHMRC funding ID 628875), in a strategic partnership with The University of Melbourne, The Centre for Human Psychopharmacology at the Swinburne University of Technology. Jerome Sarris, Erica McIntyre and David A. Camfield have no conflicts of interest that are directly relevant to the content of this article. 1 Abstract Research in the area of herbal psychopharmacology has revealed a variety of promising medicines that may provide benefit in the treatment of general anxiety and specific anxiety disorders. However, a comprehensive review of plant-based anxiolytics has been absent to date. Thus, our aim was to provide a comprehensive narrative review of plant-based medicines that have clinical and/or preclinical evidence of anxiolytic activity. We present the article in two parts. In part one, we reviewed herbal medicines for which only preclinical investigations for anxiolytic activity have been performed. In this current article (part two), we review herbal medicines for which there have been both preclinical and clinical investigations for anxiolytic activity. -

Kava Kava Extract Is Available from Ashland Chemical Co., Mini Star International, Inc., and QBI (Quality Botanical Ingredients, Inc.)
SUMMARY OF DATA FOR CHEMICAL SELECTION Kava Kava 9000-38-8; 84696-40-2 November 1998 TABLE OF CONTENTS Basis for Nomination Chemical Identification Production Information Use Pattern Human Exposure Regulatory Status Evidence for Possible Carcinogenic Activity Human Data Animal Data Metabolism Other Biological Effects Structure-Activity Relationships References BASIS OF NOMINATION TO THE CSWG Kava kava is brought to the attention of the CSWG because it is a rapidly growing, highly used dietary supplement introduced into the mainstream U.S. market relatively recently. Through this use, millions of consumers using antianxiety preparations are potentially exposed to kava kava. A traditional beverage of various Pacific Basin countries, kava clearly has psychoactive properties. The effects of its long-term consumption have not been documented adequately; preliminary studies suggest possibly serious organ system effects. The potential carcinogenicity of kava and its principal constituents are unknown. INPUT FROM GOVERNMENT AGENCIES/INDUSTRY The U.S. Pharmacopeia is in the process of reviewing kava kava. No decision on preparation of a monograph has been made. SELECTION STATUS ACTION BY CSWG: 12/14/98 Studies requested: - Toxicological evaluation, to include studies of reproductive toxicity and neurotoxicity - Genotoxicity Priority: High Rationale/Remarks: - Significant human exposure - Leading dietary supplement with rapidly growing use - Concern that kava has been promoted as a substitute for ritilin in children - Test extract standardized to 30 percent kavalactones - NCI is conducting studies in Salmonella typhimurium CHEMICAL IDENTIFICATION CAS Registry Number: 9000-38-8 Kava-kava resin (8CI) Chemical Abstract Service Name: 84696-40-2 CAS Registry Number: Pepper (Piper), P. methysticum, ext. Chemical Abstract Service Name: Extract of kava; kava extract; Piper Synonyms and Trade Names: methisticum extract Description: The tropical shrub Piper methysticum is widely cultivated in the South Pacific. -

Current Perspectives in Herbal and Conventional Drug Interactions
Surana et al. Future Journal of Pharmaceutical Sciences (2021) 7:103 Future Journal of https://doi.org/10.1186/s43094-021-00256-w Pharmaceutical Sciences REVIEW Open Access Current perspectives in herbal and conventional drug interactions based on clinical manifestations Ajaykumar Rikhabchand Surana* , Shivam Puranmal Agrawal, Manoj Ramesh Kumbhare and Snehal Balu Gaikwad Abstract Background: Herbs are an important source of pharmaceuticals. Herbs are traditionally used by millions of peoples for medicine, food and drink in developed and developing nations considering that they are safe. But, interaction of herbs with other medicines may cause serious adverse effects or reduces their efficacy. The demand for “alternative” medicines has been increased significantly, which include medicine derived from plant or herbal origin. The objective of this review article mainly focuses on drug interactions of commonly used herbs along with possible mechanisms. The method adopted for this review is searching of herb-drug interactions in online database. Main text: Herb-drug interaction leads to pharmacological modification. The drug use along with herbs may show pharmacodynamic and pharmacokinetic interactions. Pharmacokinetic interaction causes alteration in absorption, distribution, metabolism and elimination. Similarly, pharmacodynamic interaction causes additive or synergistic or antagonist effect on the drugs or vice versa. Researchers had demonstrated that herbs show the toxicities and drug interactions like other pharmacologically active compounds. There is lack of knowledge amongst physician, pharmacist and consumers related to pharmacological action and mechanism of herb-drug interaction. This review article focuses on the herb-drug interaction of danshen (Salvia miltiorrhiza), Echinacea (Echinacea purpurea), garlic (Allium sativum), ginkgo (Ginkgo biloba), goldenseal (Hydrastis canadensis), green tea (Camellia sinensis), kava (Piper methysticum), liquorice (Glycyrrhiza glabra), milk thistle (Silybum marianum) and St. -

Kava As a Pharmacotherapy of Anxiety
l ch cina em di is Rivers et al., Med chem 2016, 6:2 e tr M y Medicinal chemistry DOI: 10.4172/2161-0444.1000329 ISSN: 2161-0444 Review Article Open Access Kava as a Pharmacotherapy of Anxiety Disorders: Promises and Concerns Zachary Rivers1, Chengguo Xing2 and Sreekanth Narayanapillai2* 1College of Pharmacy, University of Minnesota, 308 Harvard St SE, Minneapolis, MN 55455, USA 2Department of Medicinal Chemistry, College of Pharmacy, University of Minnesota, 2231 6th St SE, Minneapolis, MN 55455, USA Abstract Current standard pharmacotherapies for anxiety management come with a host of side-effects that may deter the patients from utilizing them. Kava, a traditional beverage from the South Pacific region, has been used as a natural medicine for centuries and has been hypothesized to contain anxiolytic properties. There are a few well-designed, randomly controlled trials that have evaluated the effectiveness of kava or its constituents against anxiety disorders. They have generally shown kava to be effective in managing the disease. However, there has been a serious concern about the hepatotoxic risk of kava, which greatly limits its anxiolytic development and application. This review attempts to summarize the recent anxiolytic trials using kava, the associated hepatotoxicity risks, the potential responsible chemicals for these two activities, and the mechanisms of action. Overall, kava has a great potential to be developed as a natural anxiolytic agent through a systematic approach, but the present form should be used with caution. Keywords: Anxiety disorders; Muscle tension; Insomnia; Treatment conditions than a prescription drug. Kava is one such natural product that has been used in the treatment of anxiety disorders. -

Article – – = = = = C7-C8 – – – – Is Known to = = C5-C6
J. Braz. Chem. Soc., Vol. 20, No. 9, 1687-1697, 2009. Printed in Brazil - ©2009 Sociedade Brasileira de Química 0103 - 5053 $6.00+0.00 Article Synthesis of Novel Kavain-like Derivatives and Evaluation of their Cytotoxic Activity Patricia de A. Amaral,a,b Julien Petrignet,c Nicolas Gouault,b Taciane Agustini,a Françoise Lohézic-Ledévéhat,b Alexandre Cariou,b René Grée,c Vera L. Eifler-Lima*,a and Michèle Davidb aLaboratório de Síntese Orgânica Medicinal, Faculdade de Farmácia, Universidade Federal do Rio Grande do Sul, 90610-000 Porto Alegre-RS, Brazil bLaboratoire de Chimie Therapeutique, UPRES 4090, Université de Rennes 1, Rennes, France cLaboratoire de Chimie et Photonique Moléculaires, CNRS UMR 6510, Université de Rennes 1, Rennes, France Reações de acoplamento do tipo Heck, Sonogashira-Hagihara, Suzuki-Miyaura e reação de aldolisação catalizadas por metal foram utilizadas para a obtenção de três séries de d-valerolactonas substituídas em posições 3, 4, 5 e 6 do anel lactônico. As 26 d-valerolactonas sintetizadas foram testadas contra três linhagens celulares e cinco delas exibiram uma moderada atividade citotóxica. Palladium-catalyzed cross coupling reactions (Sonogashira-Hagihara, Suzuki-Miyaura, and Heck) coupling and nickel hydride-mediated tandem isomerization aldolisation have been used for the synthesis of three series of d-valerolactones substituted in positions 3, 4, 5 and 6 of the lactone ring. The 26 kavaïn-like derivatives were tested against three cell lines and five of them exhibited a weak cytotoxic activity. Keywords: -

Phytochem Referenzsubstanzen
High pure reference substances Phytochem Hochreine Standardsubstanzen for research and quality für Forschung und management Referenzsubstanzen Qualitätssicherung Nummer Name Synonym CAS FW Formel Literatur 01.286. ABIETIC ACID Sylvic acid [514-10-3] 302.46 C20H30O2 01.030. L-ABRINE N-a-Methyl-L-tryptophan [526-31-8] 218.26 C12H14N2O2 Merck Index 11,5 01.031. (+)-ABSCISIC ACID [21293-29-8] 264.33 C15H20O4 Merck Index 11,6 01.032. (+/-)-ABSCISIC ACID ABA; Dormin [14375-45-2] 264.33 C15H20O4 Merck Index 11,6 01.002. ABSINTHIN Absinthiin, Absynthin [1362-42-1] 496,64 C30H40O6 Merck Index 12,8 01.033. ACACETIN 5,7-Dihydroxy-4'-methoxyflavone; Linarigenin [480-44-4] 284.28 C16H12O5 Merck Index 11,9 01.287. ACACETIN Apigenin-4´methylester [480-44-4] 284.28 C16H12O5 01.034. ACACETIN-7-NEOHESPERIDOSIDE Fortunellin [20633-93-6] 610.60 C28H32O14 01.035. ACACETIN-7-RUTINOSIDE Linarin [480-36-4] 592.57 C28H32O14 Merck Index 11,5376 01.036. 2-ACETAMIDO-2-DEOXY-1,3,4,6-TETRA-O- a-D-Glucosamine pentaacetate 389.37 C16H23NO10 ACETYL-a-D-GLUCOPYRANOSE 01.037. 2-ACETAMIDO-2-DEOXY-1,3,4,6-TETRA-O- b-D-Glucosamine pentaacetate [7772-79-4] 389.37 C16H23NO10 ACETYL-b-D-GLUCOPYRANOSE> 01.038. 2-ACETAMIDO-2-DEOXY-3,4,6-TRI-O-ACETYL- Acetochloro-a-D-glucosamine [3068-34-6] 365.77 C14H20ClNO8 a-D-GLUCOPYRANOSYLCHLORIDE - 1 - High pure reference substances Phytochem Hochreine Standardsubstanzen for research and quality für Forschung und management Referenzsubstanzen Qualitätssicherung Nummer Name Synonym CAS FW Formel Literatur 01.039. -

(12) United States Patent (10) Patent No.: US 6,746,695 B1 Martin Et Al
USOO6746695B1 (12) United States Patent (10) Patent No.: US 6,746,695 B1 Martin et al. (45) Date of Patent: Jun. 8, 2004 (54) PHARMACEUTICAL PREPARATIONS OF Abad, MJ et al., Anti-inflammatory activity of some medici BOACTIVE SUBSTANCES EXTRACTED nal plant extracts from Venezuela. J. Ethnopharmacol1996 FROM NATURAL SOURCES Dec;55(1):63–8. Alarcon-Aguilara, FJ, et al., Study of the anti-hyperglyce (75) Inventors: Michael Z. Martin, Laupahoehoe, HI mic effect of plants used as antidiabetics. J. Ethnopharmacol (US); Mehdi Ashraf-Khorassani, Blacksburg, VA (US); Larry Taylor, 612:101-110 (1998). Blacksburg, VA (US) Almeida CE et al., Analysis of anti-diarrheic effect of plants used in popular medicine. Rev Saude Publica 1995 (73) Assignees: Armadillo Pharmaceuticals, Inc., Dec;29(6):428-33. Armocas, CA (US); Virginia Tech. Alves KB, et al., Inhibition of aminopeptidase activity by Intellectual Properties, Inc., aromatic and other cyclic compounds. Braz, J Med Biol Res. Blackburg, VA (US) 1992:25(11):1103–6. (*) Notice: Subject to any disclaimer, the term of this Anesini C, et al., Screening of plants used in Argentine folk patent is extended or adjusted under 35 medicine for anti-microbial activity. J Ethnopharmacol. U.S.C. 154(b) by 0 days. 1993 Jun;39(2):119–28. Arletti, R, et al., Stimulating property of Turnera diffusa and Pfafia paniculata eXtracts on the Sexual behavior of male (21) Appl. No.: 09/578,849 rats, Psychopharmacology (Berl). 1999 Mar;143(1): 15-9. (22) Filed: May 26, 2000 Auterhoff, Het al., Constituents of the drug Damiana. Arch Related U.S. -

Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Sedative
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Chemicals for Sedative Chemical Dosage (+)-BORNYL-ISOVALERATE -- (-)-DICENTRINE LD50=187 1,8-CINEOLE -- 2-METHYLBUT-3-ENE-2-OL -- 6-GINGEROL -- 6-SHOGAOL -- ACYLSPINOSIN -- ADENOSINE -- AKUAMMIDINE -- ALPHA-PINENE -- ALPHA-TERPINEOL -- AMYL-BUTYRATE -- AMYLASE -- ANEMONIN -- ANGELIC-ACID -- ANGELICIN ED=20-80 ANISATIN 0.03 mg/kg ANNOMONTINE -- APIGENIN 30-100 mg/kg ARECOLINE 1 mg/kg ASARONE -- ASCARIDOLE -- ATHEROSPERMINE -- BAICALIN -- BALDRINAL -- BENZALDEHYDE -- BENZYL-ALCOHOL -- Chemical Dosage BERBERASTINE -- BERBERINE -- BERGENIN -- BETA-AMYRIN-PALMITATE -- BETA-EUDESMOL -- BETA-PHENYLETHANOL -- BETA-RESERCYCLIC-ACID -- BORNEOL -- BORNYL-ACETATE -- BOSWELLIC-ACID 20-55 mg/kg ipr rat BRAHMINOSIDE -- BRAHMOSIDE -- BULBOCAPNINE -- BUTYL-PHTHALIDE -- CAFFEIC-ACID 500 mg CANNABIDIOLIC-ACID -- CANNABINOL ED=200 CARPACIN -- CARVONE -- CARYOPHYLLENE -- CHELIDONINE -- CHIKUSETSUSAPONIN -- CINNAMALDEHYDE -- CITRAL ED 1-32 mg/kg CITRAL 1 mg/kg CITRONELLAL ED=1 mg/kg CITRONELLOL -- 2 Chemical Dosage CODEINE -- COLUBRIN -- COLUBRINOSIDE -- CORYDINE -- CORYNANTHEINE -- COUMARIN -- CRYOGENINE -- CRYPTOCARYALACTONE 250 mg/kg CUMINALDEHYDE -- CUSSONOSIDE-A -- CYCLOSTACHINE-A -- DAIGREMONTIANIN -- DELTA-9-THC 10 mg/orl/man/day DESERPIDINE -- DESMETHOXYANGONIN 200 mg/kg ipr DIAZEPAM 40-200 ug/lg/3-4x/day DICENTRINE LD50=187 DIDROVALTRATUM -- DIHYDROKAWAIN -- DIHYDROMETHYSTICIN 60 mg/kg ipr DIHYDROVALTRATE -- DILLAPIOL ED50=1.57 DIMETHOXYALLYLBENZENE -- DIMETHYLVINYLCARBINOL -- DIPENTENE