1 DUX4 Regulates Oocyte to Embryo Transition in Human 1 2 Sanna
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Small Cell Ovarian Carcinoma: Genomic Stability and Responsiveness to Therapeutics
Gamwell et al. Orphanet Journal of Rare Diseases 2013, 8:33 http://www.ojrd.com/content/8/1/33 RESEARCH Open Access Small cell ovarian carcinoma: genomic stability and responsiveness to therapeutics Lisa F Gamwell1,2, Karen Gambaro3, Maria Merziotis2, Colleen Crane2, Suzanna L Arcand4, Valerie Bourada1,2, Christopher Davis2, Jeremy A Squire6, David G Huntsman7,8, Patricia N Tonin3,4,5 and Barbara C Vanderhyden1,2* Abstract Background: The biology of small cell ovarian carcinoma of the hypercalcemic type (SCCOHT), which is a rare and aggressive form of ovarian cancer, is poorly understood. Tumourigenicity, in vitro growth characteristics, genetic and genomic anomalies, and sensitivity to standard and novel chemotherapeutic treatments were investigated in the unique SCCOHT cell line, BIN-67, to provide further insight in the biology of this rare type of ovarian cancer. Method: The tumourigenic potential of BIN-67 cells was determined and the tumours formed in a xenograft model was compared to human SCCOHT. DNA sequencing, spectral karyotyping and high density SNP array analysis was performed. The sensitivity of the BIN-67 cells to standard chemotherapeutic agents and to vesicular stomatitis virus (VSV) and the JX-594 vaccinia virus was tested. Results: BIN-67 cells were capable of forming spheroids in hanging drop cultures. When xenografted into immunodeficient mice, BIN-67 cells developed into tumours that reflected the hypercalcemia and histology of human SCCOHT, notably intense expression of WT-1 and vimentin, and lack of expression of inhibin. Somatic mutations in TP53 and the most common activating mutations in KRAS and BRAF were not found in BIN-67 cells by DNA sequencing. -

CD56+ T-Cells in Relation to Cytomegalovirus in Healthy Subjects and Kidney Transplant Patients
CD56+ T-cells in Relation to Cytomegalovirus in Healthy Subjects and Kidney Transplant Patients Institute of Infection and Global Health Department of Clinical Infection, Microbiology and Immunology Thesis submitted in accordance with the requirements of the University of Liverpool for the degree of Doctor in Philosophy by Mazen Mohammed Almehmadi December 2014 - 1 - Abstract Human T cells expressing CD56 are capable of tumour cell lysis following activation with interleukin-2 but their role in viral immunity has been less well studied. The work described in this thesis aimed to investigate CD56+ T-cells in relation to cytomegalovirus infection in healthy subjects and kidney transplant patients (KTPs). Proportions of CD56+ T cells were found to be highly significantly increased in healthy cytomegalovirus-seropositive (CMV+) compared to cytomegalovirus-seronegative (CMV-) subjects (8.38% ± 0.33 versus 3.29%± 0.33; P < 0.0001). In donor CMV-/recipient CMV- (D-/R-)- KTPs levels of CD56+ T cells were 1.9% ±0.35 versus 5.42% ±1.01 in D+/R- patients and 5.11% ±0.69 in R+ patients (P 0.0247 and < 0.0001 respectively). CD56+ T cells in both healthy CMV+ subjects and KTPs expressed markers of effector memory- RA T-cells (TEMRA) while in healthy CMV- subjects and D-/R- KTPs the phenotype was predominantly that of naïve T-cells. Other surface markers, CD8, CD4, CD58, CD57, CD94 and NKG2C were expressed by a significantly higher proportion of CD56+ T-cells in healthy CMV+ than CMV- subjects. Functional studies showed levels of pro-inflammatory cytokines IFN-γ and TNF-α, as well as granzyme B and CD107a were significantly higher in CD56+ T-cells from CMV+ than CMV- subjects following stimulation with CMV antigens. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Structural Basis of UGUA Recognition by the Nudix Protein CFI 25
Structural basis of UGUA recognition by the Nudix protein CFIm25 and implications for a regulatory role in mRNA 3′ processing Qin Yang, Gregory M. Gilmartin1, and Sylvie Doublié1 Department of Microbiology and Molecular Genetics, Stafford Hall, University of Vermont, Burlington, VT 05405 Edited by Joan A. Steitz, Howard Hughes Medical Institute, New Haven, CT, and approved April 20, 2010 (received for review January 21, 2010) — Human Cleavage Factor Im (CFIm) is an essential component of the C-terminal RS/RD alternating charge domain a structure simi- pre-mRNA 3′ processing complex that functions in the regulation of lar to that of the SR-protein family of splicing regulators. The 25 poly(A) site selection through the recognition of UGUA sequences small subunit (CFIm ) contains a Nudix domain, a protein do- upstream of the poly(A) site. Although the highly conserved 25 kDa main that most often participates in the hydrolysis of substrates subunit (CFIm25) of the CFIm complex possesses a characteristic containing a nucleotide diphosphate linked to a variable moiety α β α / / Nudix fold, CFIm25 has no detectable hydrolase activity. Here X (15). Found throughout all three kingdoms, Nudix proteins we report the crystal structures of the human CFIm25 homodimer in participate in a wide range of crucial housekeeping functions, in- complex with UGUAAA and UUGUAU RNA sequences. CFIm25 is the cluding the hydrolysis of mutagenic nucleotides, the modulation first Nudix protein to be reported to bind RNA in a sequence- of the levels of signaling molecules, and the monitoring of meta- 25 α β specific manner. The UGUA sequence contributes to binding speci- bolic intermediates (15). -

CPSF6 Links Alternative Polyadenylation to Metabolism
Tan et al. Journal of Experimental & Clinical Cancer Research (2021) 40:85 https://doi.org/10.1186/s13046-021-01884-z RESEARCH Open Access CPSF6 links alternative polyadenylation to metabolism adaption in hepatocellular carcinoma progression Sheng Tan1†, Ming Zhang2†, Xinglong Shi3†, Keshuo Ding4, Qiang Zhao3, Qianying Guo4, Hao Wang4, Zhengsheng Wu4, Yani Kang3, Tao Zhu5*, Jielin Sun1* and Xiaodong Zhao1* Abstract Background: Alternative polyadenylation (APA) is an important mechanism of gene expression regulation through generation of RNA isoforms with distinct 3′ termini. Increasing evidence has revealed that APA is actively involved in development and disease, including hepatocellular carcinoma (HCC). However, how APA functions in tumor formation and progression remains elusive. In this study, we investigated the role of cleavage factor I (CFIm) subunit CPSF6 in human hepatocellular carcinoma (HCC). Methods: Expression levels of CPSF6 in clinical tissues and cell lines were determined by qRT-PCR and western blot. Functional assays, including the cell number, MTT, colony formation and transwell, were used to determine the oncogenic role of CPSF6 in HCC. Animal experiments were used to determine the role of CPSF6 in HCC tumorigenicity in vivo. Deep sequencing-based 3 T-seq was used to profile the transcriptome-wide APA sites in both HCC cells and CPSF6 knockdown HCC cells. The function of CPSF6-affected target NQO1 with distinct 3′UTRs was characterized by metabolism assays. Results: We observed CPSF6 was upregulated in HCC and the high expression of CPSF6 was associated with poor prognosis in patients. Overexpression of CPSF6 promoted proliferation, migration and invasion of HCC cells in vitro and in vivo. -

Downloads/ (Accessed on 17 January 2020)
cells Review Novel Approaches for Identifying the Molecular Background of Schizophrenia Arkadiy K. Golov 1,2,*, Nikolay V. Kondratyev 1 , George P. Kostyuk 3 and Vera E. Golimbet 1 1 Mental Health Research Center, 34 Kashirskoye shosse, 115522 Moscow, Russian; [email protected] (N.V.K.); [email protected] (V.E.G.) 2 Institute of Gene Biology, Russian Academy of Sciences, 34/5 Vavilova Street, 119334 Moscow, Russian 3 Alekseev Psychiatric Clinical Hospital No. 1, 2 Zagorodnoye shosse, 115191 Moscow, Russian; [email protected] * Correspondence: [email protected] Received: 5 November 2019; Accepted: 16 January 2020; Published: 18 January 2020 Abstract: Recent advances in psychiatric genetics have led to the discovery of dozens of genomic loci associated with schizophrenia. However, a gap exists between the detection of genetic associations and understanding the underlying molecular mechanisms. This review describes the basic approaches used in the so-called post-GWAS studies to generate biological interpretation of the existing population genetic data, including both molecular (creation and analysis of knockout animals, exploration of the transcriptional effects of common variants in human brain cells) and computational (fine-mapping of causal variability, gene set enrichment analysis, partitioned heritability analysis) methods. The results of the crucial studies, in which these approaches were used to uncover the molecular and neurobiological basis of the disease, are also reported. Keywords: schizophrenia; GWAS; causal genetic variants; enhancers; brain epigenomics; genome/epigenome editing 1. Introduction Schizophrenia is a severe mental illness that affects between 0.5% and 0.7% of the human population [1]. Both environmental and genetic factors are thought to be involved in its pathogenesis, with genetic factors playing a key role in disease risk, as the heritability of schizophrenia is estimated to be 70–85% [2,3]. -

Whole Exome Sequencing in Families at High Risk for Hodgkin Lymphoma: Identification of a Predisposing Mutation in the KDR Gene
Hodgkin Lymphoma SUPPLEMENTARY APPENDIX Whole exome sequencing in families at high risk for Hodgkin lymphoma: identification of a predisposing mutation in the KDR gene Melissa Rotunno, 1 Mary L. McMaster, 1 Joseph Boland, 2 Sara Bass, 2 Xijun Zhang, 2 Laurie Burdett, 2 Belynda Hicks, 2 Sarangan Ravichandran, 3 Brian T. Luke, 3 Meredith Yeager, 2 Laura Fontaine, 4 Paula L. Hyland, 1 Alisa M. Goldstein, 1 NCI DCEG Cancer Sequencing Working Group, NCI DCEG Cancer Genomics Research Laboratory, Stephen J. Chanock, 5 Neil E. Caporaso, 1 Margaret A. Tucker, 6 and Lynn R. Goldin 1 1Genetic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; 2Cancer Genomics Research Laboratory, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; 3Ad - vanced Biomedical Computing Center, Leidos Biomedical Research Inc.; Frederick National Laboratory for Cancer Research, Frederick, MD; 4Westat, Inc., Rockville MD; 5Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; and 6Human Genetics Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD, USA ©2016 Ferrata Storti Foundation. This is an open-access paper. doi:10.3324/haematol.2015.135475 Received: August 19, 2015. Accepted: January 7, 2016. Pre-published: June 13, 2016. Correspondence: [email protected] Supplemental Author Information: NCI DCEG Cancer Sequencing Working Group: Mark H. Greene, Allan Hildesheim, Nan Hu, Maria Theresa Landi, Jennifer Loud, Phuong Mai, Lisa Mirabello, Lindsay Morton, Dilys Parry, Anand Pathak, Douglas R. Stewart, Philip R. Taylor, Geoffrey S. Tobias, Xiaohong R. Yang, Guoqin Yu NCI DCEG Cancer Genomics Research Laboratory: Salma Chowdhury, Michael Cullen, Casey Dagnall, Herbert Higson, Amy A. -

Supplemental Material 1
SUP. FIGURE S1 DAPI NFAT Merge DMSO Ac5SGlcNAc Figure S1. Inhibition of OGT does not prevent nuclear translocation of NFAT. Jurkat cells !"#$%&'()*+(!!,-.'/012"#..(3'4056'7(+('"+(#"(3'7,"8'9:';<'5=29>/%=45='?+'@<>A'B?+'CD' 8+!E''F(%%!'7(+('"8(-'*%#"(3'?-'#-",2F@GHF@ID2=?#"(3'=?J(+!%,*!'B?+'G:'K,-!E''5B"(+'B,)#",?-L' =(%%!'7(+('#-#%&M(3'$&'=?-B?=#%'K,=+?!=?*&E SUP. FIGURE S2 Labeled Unlabeled Labeled Unlabeled 1h 18h Az PEG 1h 19h Az PEG !"#$%"#&'( + + + + !"#$%"#&'( + + + + 110 EWSR1 160 *34+&5 80 &,/ 160 )*+-2$ 160 110 )*+,& 80 60 &,/ 160 110 80 0"1"- RUNX1 110 80 60 60 50 160 SP1 160 110 ELF1 110 80 160 NUP98 110 80 )*+&-. &,/ Figure S2. PEG mass tags allow visualization of OGlcNAc stoichiometry. 5K PEG mass tags were affixed to OGlcNAc groups on proteins from control or activated T cells via enzy matic labeling with azide and copperfree click chemistry. Proteins were then analyzed for shifts in electrophoretic mobility by immunoblot. For the unlabeled control samples, either the azide (Az) or PEG (PEG) reagent was omitted during the labeling procedure. Note that HCFC1 appears as multiple bands because the protein is expressed as a single polypeptide that under goes proteolytic processing. UBAP2L appears as two bands in the unlabeled control samples due to alternative splicing. Table S1. Details of 133 higher confidence and 81 lower confidence O-GlcNAc glycoproteins1. Confidence Uniprot ID Symbol Specificity Chi Higher P55265 ADAR 100% 9.11E-04 Higher Q09666 AHNAK 92% 2.36E-16 Higher Q8IWZ3 ANKHD1 100% 1.19E-03 Higher -

Introducing Gene Deletions by Mouse Zygote Electroporation of Cas12a/Cpf1
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2019 Introducing gene deletions by mouse zygote electroporation of Cas12a/Cpf1 Dumeau, Charles-Etienne ; Monfort, Asun ; Kissling, Lucas ; Swarts, Daan C ; Jinek, Martin ; Wutz, Anton Abstract: CRISPR-associated (Cas) nucleases are established tools for engineering of animal genomes. These programmable RNA-guided nucleases have been introduced into zygotes using expression vectors, mRNA, or directly as ribonucleoprotein (RNP) complexes by different delivery methods. Whereas mi- croinjection techniques are well established, more recently developed electroporation methods simplify RNP delivery but can provide less consistent efficiency. Previously, we have designed Cas12a-crRNA pairs to introduce large genomic deletions in the Ubn1, Ubn2, and Rbm12 genes in mouse embryonic stem cells (ESC). Here, we have optimized the conditions for electroporation of the same Cas12a RNP pairs into mouse zygotes. Using our protocol, large genomic deletions can be generated efficiently by electroporation of zygotes with or without an intact zona pellucida. Electroporation of as few as ten zygotes is sufficient to obtain a gene deletion in mice suggesting potential applicability of thismethod for species with limited availability of zygotes. DOI: https://doi.org/10.1007/s11248-019-00168-9 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-181145 Journal Article Published Version The following work is licensed under a Creative Commons: Attribution 4.0 International (CC BY 4.0) License. Originally published at: Dumeau, Charles-Etienne; Monfort, Asun; Kissling, Lucas; Swarts, Daan C; Jinek, Martin; Wutz, Anton (2019). -
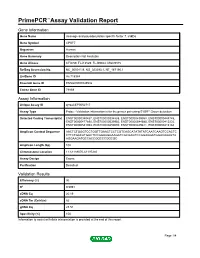
Primepcr™Assay Validation Report
PrimePCR™Assay Validation Report Gene Information Gene Name cleavage and polyadenylation specific factor 7, 59kDa Gene Symbol CPSF7 Organism Human Gene Summary Description Not Available Gene Aliases CFIm59, FLJ12529, FLJ39024, MGC9315 RefSeq Accession No. NC_000011.9, NG_023393.1, NT_167190.1 UniGene ID Hs.718984 Ensembl Gene ID ENSG00000149532 Entrez Gene ID 79869 Assay Information Unique Assay ID qHsaCEP0052717 Assay Type Probe - Validation information is for the primer pair using SYBR® Green detection Detected Coding Transcript(s) ENST00000340437, ENST00000394888, ENST00000439958, ENST00000448745, ENST00000477890, ENST00000539952, ENST00000544585, ENST00000413232, ENST00000541963, ENST00000450000, ENST00000449811, ENST00000413184 Amplicon Context Sequence AACTCTGGGTCCTGGTTGAACTCCTCGTCAGCATATATATCAATCAAGTCCACTC CTTCTGACATGGCTCCGGAAGGAAGATCGCGAGTCCGGAGGATGGACAAAGTA AGGAAGATGCCACCGGCCCGGCGC Amplicon Length (bp) 102 Chromosome Location 11:61189070-61197240 Assay Design Exonic Purification Desalted Validation Results Efficiency (%) 96 R2 0.9991 cDNA Cq 20.19 cDNA Tm (Celsius) 82 gDNA Cq 28.51 Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 1/4 PrimePCR™Assay Validation Report CPSF7, Human Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak Melt curve analysis of above amplification Standard Curve Standard curve generated using 20 million copies of template diluted 10-fold to 20 copies Page 2/4 PrimePCR™Assay Validation Report Products used to generate validation data Real-Time PCR Instrument CFX384 Real-Time PCR Detection System Reverse Transcription Reagent iScript™ Advanced cDNA Synthesis Kit for RT-qPCR Real-Time PCR Supermix SsoAdvanced™ SYBR® Green Supermix Experimental Sample qPCR Human Reference Total RNA Data Interpretation Unique Assay ID This is a unique identifier that can be used to identify the assay in the literature and online. -

Chromosomal Microarray Analysis in Turkish Patients with Unexplained Developmental Delay and Intellectual Developmental Disorders
177 Arch Neuropsychitry 2020;57:177−191 RESEARCH ARTICLE https://doi.org/10.29399/npa.24890 Chromosomal Microarray Analysis in Turkish Patients with Unexplained Developmental Delay and Intellectual Developmental Disorders Hakan GÜRKAN1 , Emine İkbal ATLI1 , Engin ATLI1 , Leyla BOZATLI2 , Mengühan ARAZ ALTAY2 , Sinem YALÇINTEPE1 , Yasemin ÖZEN1 , Damla EKER1 , Çisem AKURUT1 , Selma DEMİR1 , Işık GÖRKER2 1Faculty of Medicine, Department of Medical Genetics, Edirne, Trakya University, Edirne, Turkey 2Faculty of Medicine, Department of Child and Adolescent Psychiatry, Trakya University, Edirne, Turkey ABSTRACT Introduction: Aneuploids, copy number variations (CNVs), and single in 39 (39/123=31.7%) patients. Twelve CNV variant of unknown nucleotide variants in specific genes are the main genetic causes of significance (VUS) (9.75%) patients and 7 CNV benign (5.69%) patients developmental delay (DD) and intellectual disability disorder (IDD). were reported. In 6 patients, one or more pathogenic CNVs were These genetic changes can be detected using chromosome analysis, determined. Therefore, the diagnostic efficiency of CMA was found to chromosomal microarray (CMA), and next-generation DNA sequencing be 31.7% (39/123). techniques. Therefore; In this study, we aimed to investigate the Conclusion: Today, genetic analysis is still not part of the routine in the importance of CMA in determining the genomic etiology of unexplained evaluation of IDD patients who present to psychiatry clinics. A genetic DD and IDD in 123 patients. diagnosis from CMA can eliminate genetic question marks and thus Method: For 123 patients, chromosome analysis, DNA fragment analysis alter the clinical management of patients. Approximately one-third and microarray were performed. Conventional G-band karyotype of the positive CMA findings are clinically intervenable. -

Joint Profiling of Chromatin Accessibility and CAR-T Integration Site Analysis at Population and Single-Cell Levels
Joint profiling of chromatin accessibility and CAR-T integration site analysis at population and single-cell levels Wenliang Wanga,b,c,d, Maria Fasolinoa,b,c,d, Benjamin Cattaua,b,c,d, Naomi Goldmana,b,c,d, Weimin Konge,f,g, Megan A. Fredericka,b,c,d, Sam J. McCrighta,b,c,d, Karun Kiania,b,c,d, Joseph A. Fraiettae,f,g,h, and Golnaz Vahedia,b,c,d,f,1 aDepartment of Genetics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104; bInstitute for Immunology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104; cEpigenetics Institute, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104; dInstitute for Diabetes, Obesity and Metabolism, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104; eDepartment of Microbiology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104; fAbramson Family Cancer Center, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104; gCenter for Cellular Immunotherapies, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104; and hParker Institute for Cancer Immunotherapy, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104 Edited by Anjana Rao, La Jolla Institute for Allergy and Immunology, La Jolla, CA, and approved January 30, 2020 (received for review November 3, 2019) Chimeric antigen receptor (CAR)-T immunotherapy has yielded tumor killing. To determine the extent to which these two sce- impressive results in several B cell malignancies, establishing itself narios occur in vivo, it is essential to simultaneously determine as a powerful means to redirect the natural properties of T lym- T cell fate and map where CAR-T vectors integrate into the phocytes.