Comprehensive Management of Pelvic Floor Dysfunction in Women
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pelvic Floor Dysfunction UPDATE
Pelvic floor dysfunction UPDATE A. Rebecca Meekins, MD Cindy L. Amundsen, MD Dr. Meekins is a Fellow in Female Pelvic Dr. Amundsen is Roy T. Parker Professor in Medicine and Reconstructive Surgery, Obstetrics and Gynecology, Urogynecology Department of Obstetrics and Gynecology, and Reconstructive Pelvic Surgery; Associate Duke University School of Medicine, Professor of Surgery, Division of Urology; Durham, North Carolina. Program Director of the Female Pelvic Medicine and Reconstructive Surgery Fellowship; Program Director of K12 Multidisciplinary Urologic Research Scholars Program; Program Director of BIRCWH, Duke University Medical Center. The authors report no financial relationships relevant to this article. “To cysto or not to cysto?” that is the ongoing question surrounding the role of cystoscopy following benign gyn surgery. These authors review data on the procedure’s ability to detect injury, an ideal method for visualizing IN THIS ureteral efflux, and how universal cystoscopy can affect the ARTICLE rate of injury. Universal cystoscopy policy sing cystoscopy to evaluate ureteral ranges from 0.02% to 2% for ureteral injury page 28 efflux and bladder integrity following and from 1% to 5% for bladder injury.5,6 U benign gynecologic surgery increases In a recent large randomized controlled the detection rate of urinary tract injuries.1 trial, the rate of intraoperative ureteral Detecting ureteral Currently, it is standard of care to perform a obstruction following uterosacral ligament obstruction cystoscopy following anti-incontinence pro- suspension (USLS) was 3.2%.7 Vaginal vault page 29 cedures, but there is no consensus among suspensions, as well as other vaginal cuff ObGyns regarding the use of universal cys- closure techniques, are common procedures Media for efflux toscopy following benign gynecologic sur- associated with urinary tract injury.8 Addi- visualization 2 gery. -

216 Does Episiotomy Influence Vaginal Resting Pressure, Pelvic Floor Muscle Strength and Endurance and Prevalence of Urinary
216 Bø K1, Hilde G2, Engh M E2 1. Norwegian School of Sport Sciences, 2. Akershus University Hospital DOES EPISIOTOMY INFLUENCE VAGINAL RESTING PRESSURE, PELVIC FLOOR MUSCLE STRENGTH AND ENDURANCE AND PREVALENCE OF URINARY INCONTINENCE SIX WEEKS POSTPARTUM? Hypothesis / aims of study Vaginal delivery is an established risk factor for weakening of the pelvic floor muscles (PFM) and development of pelvic floor dysfunctions such as urinary incontinence (UI) (1). The role of episiotomy on PFM function and prevalence of UI is debated and results differ between studies (1). The aim of the present study was to compare vaginal resting pressure (VRP), PFM strength and PFM endurance and prevalence of UI in women with and without lateral or mediolateral episiotomy six weeks postpartum. Study design, materials and methods Three hundred nulliparous pregnant women participating in a prospective cohort study giving birth at the same public hospital and able to understand the native language were recruited to the study. For this cross-sectional analysis six weeks postpartum only women with vaginal deliveries were included. Exclusion criteria were previous miscarriage after gestational week 16. Ongoing exclusion criteria were premature birth < 32 weeks, stillbirth and serious illness to mother or child. Lateral or mediolateral episiotomy was only performed on indications, e.g. foetal distress, imminent risk of severe perineal tear and in most cases when undergoing instrumental delivery. Vaginal resting pressure (cm H2O), PFM strength (cm H2O) and endurance (cm H2Osec) were assessed by a vaginal balloon connected to a high precision pressure transducer. The method has been found to be responsive, reliable and valid when used with simultaneous observation of inward movement of the perineum during contraction (2). -

Co™™I™™Ee Opinion
The American College of Obstetricians and Gynecologists WOMEN’S HEALTH CARE PHYSICIANS COMMITTEE OPINION Number 673 • September 2016 (Replaces Committee Opinion No. 345, October 2006) Committee on Gynecologic Practice This Committee Opinion was developed by the American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice and the American Society for Colposcopy and Cervical Pathology (ASCCP) in collaboration with committee member Ngozi Wexler, MD, MPH, and ASCCP members and experts Hope K. Haefner, MD, Herschel W. Lawson, MD, and Colleen K. Stockdale, MD, MS. This document reflects emerging clinical and scientific advances as of the date issued and is subject to change. The information should not be construed as dictating an exclusive course of treatment or procedure to be followed. Persistent Vulvar Pain ABSTRACT: Persistent vulvar pain is a complex disorder that frequently is frustrating to the patient and the clinician. It can be difficult to treat and rapid resolution is unusual, even with appropriate therapy. Vulvar pain can be caused by a specific disorder or it can be idiopathic. Idiopathic vulvar pain is classified as vulvodynia. Although optimal treatment remains unclear, consider an individualized, multidisciplinary approach to address all physical and emotional aspects possibly attributable to vulvodynia. Specialists who may need to be involved include sexual counselors, clinical psychologists, physical therapists, and pain specialists. Patients may perceive this approach to mean the practitioner does not believe their pain is “real”; thus, it is important to begin any treatment approach with a detailed discussion, including an explanation of the diagnosis and determination of realistic treatment goals. Future research should aim at evaluating a multimodal approach in the treatment of vulvodynia, along with more research on the etiologies of vulvodynia. -

The Role of Pelvic Floor Muscles Dysfunction in Constipation
PHYSICAL TREA MENTS January 2015 . Volume 4 . Number 4 Systematic Review: The Role of Pelvic Floor Muscles Dysfunction in Constipation Andiya Bahmani 1*, Amir Masoud Arab 1, Bijan Khorasany 2, Shabnam Shahali 1, Mojgan Foroutan 3 1. Department of Physical Therapy, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran. 2. Department of Clinical Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran. 3. Department of Educational Designing & Curriculum Planning, School of Medical Education Sciences, Shahid Beheshti University of Medical Sciences and Health Services, Tehran, Iran. Article info: A B S T R A C T Received: 10 Aug. 2014 Accepted: 27 Nov. 2014 Purpose: Pelvic floor muscle dysfunction is a common cause of constipation. This dysfunction does not respond to current treatments of constipation. Thus, it is important to identify this type of dysfunction and the role of these muscles in constipation. The purpose of the present study was to review the previously published studies concerning the role of pelvic floor muscles dysfunction in constipation and related assessment methods. Methods: Articles were obtained by searching in several databases including, Elsevier, Science Direct, ProQuest, Google scholar, and PubMed. The keywords that were used were ‘constipation,’ ‘functional constipation,’ and ‘pelvic floor dysfunction.’ Inclusion criteria included articles that were published in English from 1980 to 2013. A total of 100 articles were obtained using the mentioned keywords that among them articles about constipation, its definition, types, methods of assessment, and diagnosis were reviewed. Of these articles, 12 articles were related to the assessment procedures and pelvic floor muscle function in constipation. -

Can Pelvic Floor Injury Secondary to Delivery Be Prevented?
Int Urogynecol J DOI 10.1007/s00192-011-1530-0 REVIEWARTICLE Can pelvic floor injury secondary to delivery be prevented? Yuval Lavy & Peter K. Sand & Chava I. Kaniel & Drorith Hochner-Celnikier Received: 28 March 2011 /Accepted: 21 July 2011 # The International Urogynecological Association 2011 Abstract The number of women suffering from pelvic Introduction floor disorders (PFD) is likely to grow significantly in the coming years with a growing older population. There is an “Be fruitful and multiply” is the first commandment urgent need to investigate factors contributing to the mentioned in the Bible (Genesis 1:28). However, after development of PFD and develop preventative strategies. Eve sinned by eating the apple from the tree in the Garden We have reviewed the literature and analyzed results from of Eden, the woman was cursed as follows: “It will be with our own study regarding the association between delivery anguish that you will give birth to children” (Genesis 3:16). mode, obstetrical practice and fetal measurements, and What does the word “anguish” imply? Pain, sorrow, damage to the pelvic floor. Based on our findings, we have discomfort, or insult to the pelvic floor? suggested a flowchart helping the obstetrician to conduct In this paper, we will try to answer two questions. First, vaginal delivery with minimal pelvic floor insult. Primi- is there an association between the natural process of parity, instrumental delivery, large fetal head circumference, birthing and damage to the pelvic floor, and do we have and prolonged second stage of delivery are risk factors for enough data on the impact of delivery mode on the pelvic PFD. -
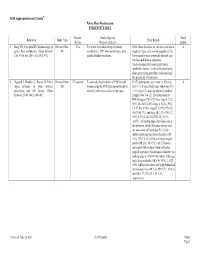
Pelvic Floor Dysfunction EVIDENCE TABLE
ACR Appropriateness Criteria® Pelvic Floor Dysfunction EVIDENCE TABLE Patients/ Study Objective Study Reference Study Type Study Results Events (Purpose of Study) Quality 1. Sung VW, Hampton BS. Epidemiology of Review/Other- N/A To review the epidemiology of urinary Pelvic floor disorders are common and have a 4 pelvic floor dysfunction. Obstet Gynecol. Dx incontinence, POP, anal incontinence, and negative impact on a woman's quality of life. Clin North Am. 2009; 36(3):421-443. painful bladder conditions. Unfortunately most women do not seek care for these debilitating symptoms. Understanding risk factors, particularly modifiable factors, is critical for developing future prevention guidelines and improving the specificity of treatments. 2. Nygaard I, Bradley C, Brandt D. Pelvic Review/Other- 270 patients To estimate the prevalence of POP in older In 270 participants, age (mean +/- SD) was 4 organ prolapse in older women: Dx women using the POP-Q examination and to 68.3 +/- 5.6 years, body mass index was 30.4 prevalence and risk factors. Obstet identify factors associated with prolapse. +/- 6.2 kg/m(2), and vaginal parity (median Gynecol. 2004; 104(3):489-497. [range]) was 3 (0-12). The proportions of POP-Q stages (95% CIs) were stage 0, 2.3% (95% CI, 0.8%-4.8%); stage I, 33.0% (95% CI, 27.4%-39.0%); stage II, 62.9% (95% CI, 56.8%-68.7%); and stage III, 1.9% (95% CI, 0.6%-4.3%). In 25.2% (95% CI, 20.1%- 30.8%), the leading edge of prolapse was at the hymen or below. -

Pelvic Floor Rehabilitation Program Pelvic Floor Rehabilitation Can Help
Pelvic Floor Rehabilitation Program Pelvic Floor Rehabilitation Can Help Physical therapy can improve pelvic floor and Inova Loudoun Hospital bladder conditions by eliminating or managing incontinence and pelvic floor conditions - giving you the confidence to live your life again. Inova Loudoun Hospital Pelvic Floor offers a comprehensive, customized approach to bladder, bowel and sexual dysfuntion. We develop a tailored plan for the evaluation and treatment of pelvic floor disorders, offering patients the following components: • EMG biofeedback • Pelvic floor exercise prescription, such as Kegel exercises, to strengthen the weakened structures around the bladder • Neuromuscular electrical stimulation • Abdominal rehabilitation • Vaginal cones and dilators • Ultrasound • Education, such as bladder training to control sudden urges to urinate • Tips on behavior modification, such as altering eating habits to relieve the symptoms of incontinence • Musculoskeletal assessment of contributing dysfunction • Manual therapy to assess and treat muscle spasms For questions or to learn more, please visit inova.org/ILHpelvicfloor or our patient navigator at 703.858.8936 G33870/3-15/400 G34063/9-15/pdf Do You Experience Any of the Following What is Pelvic Floor Dysfunction? Symptoms? • The muscles of the pelvic floor control the flow • Loss of urine with lifting, laughing, sneezing, of urine and support the bladder, uterus and running, and/or jumping rectum – organs located within the pelvis. • Increased frequency of urination, such as • For good bladder control, all parts of your urinating more than eight times a day system must work together. The pelvic floor must hold up the organs, the sphincter muscles • Sudden urgency to urinate, such as when you must control the flow of urine and the nerves hear water must activate these muscles to function. -

Female Chronic Pelvic Pain Syndromes 1 Standard of Care
BRIGHAM AND WOMEN’S HOSPITAL Department of Rehabilitation Services Physical Therapy Standard of Care: Female Chronic Pelvic Pain Syndromes ICD 9 Codes: 719.45 Pain in the pelvic region 625.9 Vulvar/pelvic pain/vulvodynia/vestibulodynia (localized provoked vestibulodynia or unprovoked) 625.0 Dyspareunia 595.1 Interstitial cystitis/painful bladder syndrome 739.5 Pelvic floor dysfunction 569.42 Anal/rectal pain 564.6 Proctalgia fugax/spasm anal sphincter 724.79 Coccygodynia 781.3 Muscular incoordination (other possible pain diagnoses: prolapse 618.0) Case Type/Diagnosis: Chronic pelvic pain (CPP) can be defined as: “non-malignant pain perceived in structures related to the pelvis, in the anterior abdominal wall below the level of the umbilicus, the spine from T10 (ovarian nerve supply) or T12 (nerve supply to pelvic musculoskeletal structures) to S5, the perineum, and all external and internal tissues within these reference zones”. 1 Specifically, pelvic pain syndrome has been further defined as: “the occurrence of persistent or recurrent episodic pelvic pain associated with symptoms suggestive of lower urinary tract, sexual, bowel or gynecological dysfunction with no proven infection or other obvious pathology”.1 Generally, female pelvic pain has been defined as pain and dysfunction in and around the pelvic outlet, specifically the suprapubic, vulvar, and anal regions. A plethora of various terms/diagnoses encompass pelvic pain as a symptom, including but not limited to: chronic pelvic pain (CPP), vulvar pain, vulvodynia, vestibulitis/vestibulodynia (localized provoked vestibulodynia or unprovoked vestibulodynia), vaginismus, dyspareunia, interstitial cystitis (IC)/painful bladder syndrome (PBS), proctalgia fugax, levator ani syndrome, pelvic floor dysfunction, vulvodynia, vestibulitis/vestibulodynia dyspareunia, vaginismus, coccygodynia, levator ani syndrome, tension myaglia of the pelvic floor, shortened pelvic floor, and muscular incoordination of the pelvic floor muscles. -

Physiotherapy for Prevention and Treatment of Fecal Incontinence in Women—Systematic Review of Methods
Journal of Clinical Medicine Review Physiotherapy for Prevention and Treatment of Fecal Incontinence in Women—Systematic Review of Methods Agnieszka Irena Mazur-Bialy 1,2,* , Daria Kołoma ´nska-Bogucka 1 , Marcin Opławski 2 and Sabina Tim 1 1 Department of Biomechanics and Kinesiology, Faculty of Health Science, Jagiellonian University Medical College, Grzegorzecka 20, 31-531 Krakow, Poland; [email protected] (D.K.-B.); [email protected] (S.T.) 2 Department of Gynecology and Obstetrics with Gynecologic Oncology, Ludwik Rydygier Memorial Specialized Hospital, Zlotej Jesieni 1, 31-826 Kraków, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-012-421-9351 Received: 18 August 2020; Accepted: 8 October 2020; Published: 12 October 2020 Abstract: Fecal incontinence (FI) affects approximately 0.25–6% of the population, both men and women. The most common causes of FI are damage to/weakness of the anal sphincter muscle and/or pelvic floor muscles, as well as neurological changes in the central or peripheral nervous system. The purpose of this study is to report the results of a systematic review of the possibilities and effectiveness of physiotherapy techniques for the prevention and treatment of FI in women. For this purpose, the PubMed, Embase, and Web of Science databases were searched for 2000–2020. A total of 22 publications qualified for detailed analysis. The studies showed that biofeedback (BF), anal sphincter muscle exercises, pelvic floor muscle training (PFMT), and electrostimulation (ES) are effective in relieving FI symptoms, as reflected in the International Continence Society recommendations (BF: level A; PFMT and ES: level B). -

An International Urogynecological Association (IUGA)
Received: 18 December 2017 | Accepted: 18 December 2017 DOI: 10.1002/nau.23508 TERMINOLOGY An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the assessment of sexual health of women with pelvic floor dysfunction Rebecca G. Rogers MD1 | Rachel N. Pauls MD2 | Ranee Thakar MD3 | Melanie Morin PhD4 | Annette Kuhn MD5 | Eckhard Petri Dd, PhD6 | Brigitte Fatton MD7 | Kristene Whitmore MD8 | Sheryl Kinsberg PhD9 | Joseph Lee MBChB, FRANZCOG10 1 Dell Medical School, University of Texas, Austin, Texas 2 TriHealth Good Samaritan Hospital, Cincinnati, Ohio 3 Croydon University Hospital Croydon, London, United Kingdom 4 Universite de Sherbrooke, Montreal, Quebec, Canada 5 University Teaching Hospital Berne (Inselspital), Bern, Switzerland 6 University of Greifswald, Schwerin, Germany 7 University Hospital Nîmes, Nimes, Languedoc-Roussillon, France 8 Drexel University College of Medicine, Philadelphia, Pennsylvania 9 Case Western Reserve University, Cleveland, Ohio 10 University of New South Wales, St Vincents Hospital, Sydney, New South Wales, Australia Correspondence Aims: The terminology in current use for sexual function and dysfunction in women Rebecca G. Rogers, Department of Women's Health, 1301 W 38th Street, with pelvic floor disorders lacks uniformity, which leads to uncertainty, confusion, Suit705, Dell Medical School, University and unintended ambiguity. The terminology for the sexual health of women with of Texas, Austin, TX 78705. pelvic floor dysfunction needs to be collated in a clinically-based consensus report. Email: [email protected] Methods: This report combines the input of members of the Standardization and Terminology Committees of two International Organizations, the International Urogynecological Association (IUGA), and the International Continence Society (ICS), assisted at intervals by many external referees. -

Understanding, Preventing and Managing Pelvic Floor Dysfunction
EDNF 2012 Conference August 2012 Women: Understanding, Preventing, and Managing Pelvic Floor Dysfunction KATHLEEN ZONARICH, PT What is Pelvic Floor Dysfunction? Pelvic floor dysfunction refers to a wide range of problems that result when pelvic floor muscles are: ¡ Weak; ¡ Tight; and/or ¡ Impaired sacroiliac joint, low back, coccyx and/or hip joint. Tissues surrounding the pelvic organs may have: ¡ Increased or decreased sensitivity; and/or ¡ Irritation resulting in pelvic pain. Pelvic Floor Dysfunction Facts Many times the underlying cause of pelvic pain is difficult to determine Pelvic floor dysfunction can cause: ¡ Incoordination in the contraction; and ¡ Relaxation of the pelvic floor muscles that assist in controlling bladder and bowel function. Pelvic floor dysfunction is not a normal course of aging All rights reserved. 1 EDNF 2012 Conference August 2012 Statistics An estimated 1/3 of all U.S. women are affected by some type of pelvic floor disorder 1 in 11 women will have pelvic floor surgery 13 million Americans are effected by incontinence ¡ Stress incontinence is most common for women ¡ Adolescent girls suffer from stress incontinence with sports Pelvic floor dysfunction occurs in women that have not given birth Structures in and around the Pelvic Floor Bones - pelvis, tailbone and sacrum Muscles Ligaments Tendons Nerves (pudendal nerve) Anatomy of the Pelvic Floor and Surrounding Structures The pelvic floor acts as a sling to: ¡ Support: ÷ Bladder ÷ Uterus ÷ Rectum ¡ Surround: ÷ Urethra ÷ Vagina ¡ Assist: ÷ With urination and defecation All rights reserved. 2 EDNF 2012 Conference August 2012 Types of Pelvic Floor Dysfunction Supportive Dysfunction ¡ A result of the loss of nerve, muscle, ligament, or fascial integrity of the pelvic floor muscles causing weakness and laxity ¡ Could be caused by injury incurred during childbearing or gynecologic surgery, chronic constipation, chronic coughing, obesity, or hormonal changes Hypertonus Dysfunction ¡ Symptoms of pain in the abdominal area, back, or vulvar region. -

Pelvic Floor Dysfunction in Primiparous Women up to 6 Months After Delivery: Cohort Study
ORIGINAL ARTICLE Pelvic floor dysfunction in primiparous women up to 6 months after delivery: cohort study Disfunções do assoalho pélvico em primíparas até 6 meses após o parto: estudo de coorte Disfunctión del suelo pélvico en primíparas hasta 6 meses después del parto: estudio de cohorte ABSTRACT Sheyla Guimarães OliveiraI Objective: To analyze pelvic floor muscular strength (PFMS), urinary (UI) and anal (AI) incontinence and dyspareunia in primiparous women up to 6 months after normal or ORCID: 0000-0002-2180-6981 cesarean delivery. Methods: this is a prospective cohort with 169 women (128 normal Adriana Caroci-BeckerII births, 41 cesarean sections), followed between 50-70 and 170-190 days postpartum, when PFMS was measured using perineometry, and UI and AI and dyspareunia, through interview. ORCID: 0000-0003-3112-8480 Results: PFMS, UI and dyspareunia were similar between types of delivery. The difference Edilaine de Paula Batista MendesIII was significant only for the time elapsed, with improvement in the studied period (2 and 6 ORCID: 0000-0002-8541-3490 months postpartum). Regarding AI, there was a significant difference between 2 and 6 months postpartum, with an interaction between type of delivery and time (p=0.022). Conclusion: Maria Luiza Gonzalez RiescoII the type of delivery did not show any influence on pelvic floor dysfunctions, except for AI. ORCID: 0000-0001-9036-5641 For all outcomes, there was an improvement in the period studied. Descriptors: After Childbirth Period; Pelvic Floor; Muscle Strength; Urinary Incontinence; Robson da Costa OliveiraIV Dyspareunia. ORCID: 0000-0001-6532-2893 Sonia Maria Junqueira Vasconcellos de OliveiraII RESUMO Objetivo: Analisar a força muscular do assoalho pélvico (FMAP), a incontinência urinária (IU) e ORCID: 0000-0002-8007-2092 anal (IA) e a dispareunia em primíparas até 6 meses após o parto normal ou cesariana.