Pathology and Laboratory Medicine Service
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Artificial Intelligence in Health Care: the Hope, the Hype, the Promise, the Peril
Artificial Intelligence in Health Care: The Hope, the Hype, the Promise, the Peril Michael Matheny, Sonoo Thadaney Israni, Mahnoor Ahmed, and Danielle Whicher, Editors WASHINGTON, DC NAM.EDU PREPUBLICATION COPY - Uncorrected Proofs NATIONAL ACADEMY OF MEDICINE • 500 Fifth Street, NW • WASHINGTON, DC 20001 NOTICE: This publication has undergone peer review according to procedures established by the National Academy of Medicine (NAM). Publication by the NAM worthy of public attention, but does not constitute endorsement of conclusions and recommendationssignifies that it is the by productthe NAM. of The a carefully views presented considered in processthis publication and is a contributionare those of individual contributors and do not represent formal consensus positions of the authors’ organizations; the NAM; or the National Academies of Sciences, Engineering, and Medicine. Library of Congress Cataloging-in-Publication Data to Come Copyright 2019 by the National Academy of Sciences. All rights reserved. Printed in the United States of America. Suggested citation: Matheny, M., S. Thadaney Israni, M. Ahmed, and D. Whicher, Editors. 2019. Artificial Intelligence in Health Care: The Hope, the Hype, the Promise, the Peril. NAM Special Publication. Washington, DC: National Academy of Medicine. PREPUBLICATION COPY - Uncorrected Proofs “Knowing is not enough; we must apply. Willing is not enough; we must do.” --GOETHE PREPUBLICATION COPY - Uncorrected Proofs ABOUT THE NATIONAL ACADEMY OF MEDICINE The National Academy of Medicine is one of three Academies constituting the Nation- al Academies of Sciences, Engineering, and Medicine (the National Academies). The Na- tional Academies provide independent, objective analysis and advice to the nation and conduct other activities to solve complex problems and inform public policy decisions. -

Job Posting Clinical Microbiology Final
The Department of Pathology & Cell Biology at Columbia University Irving Medical Center (CUIMC) is recruiting for an MD, MD/PhD, or PhD academic clinical microbiologist of any rank to join our faculty as a Medical Director of the NewYork-Presbyterian/CUIMC Clinical Microbiology Laboratory. Applicants should have an established track record of accomplishment within the field of clinical microbiology and a demonstrated ability to lead an experienced group of laboratory technologists, supervisors, and staff. In addition to strong clinical and technical skills, particular emphasis is placed on candidates with a demonstrated record of collegiality and inter-departmental collaboration. Applicants must have completed a fellowship in clinical microbiology and be board-certified/board-eligible in Medical and Public Health Microbiology through the American Board of Medical Microbiology (ABMM) or board-certified/board- eligible in Clinical Pathology with subspecialty certification in Medical Microbiology through the American Board of Pathology (ABP). The applicant must also be able to satify clinical licensing requirements to serve as a Laboratory Director in New York State. The successful applicant will help oversee diagnostic testing in the areas of Bacteriology, Virology, Mycobacteriology, Mycology, and Parasitology. The position also includes responsibilities for teaching of pathology residents, medical students, infectious diseases fellows, and technical staff. Applicants must be currently involved in ongoing research with a track record of publications in the field. The position offers a competitive salary commensurate with training and experience, and an appointment to the faculty of the Columbia University Vagelos College of Physicians & Surgeons. The Clinical Microbiology Laboratory at NewYork-Presbyterian/CUIMC is located in the Washington Heights neighborhood of New York City, offering unparalleled opportunities to work and live in a thriving, diverse, metropolitan environment with access to world-class cultural institutions, restaurants, and entertainment. -

Sports Medicine Examination Outline
Sports Medicine Examination Content I. ROLE OF THE TEAM PHYSICIAN 1% A. Ethics B. Medical-Legal 1. Physician responsibility 2. Physician liability 3. Preparticipation clearance 4. Return to play 5. Waiver of liability C. Administrative Responsibilities II. BASIC SCIENCE OF SPORTS 16% A. Exercise Physiology 1. Training Response/Physical Conditioning a.Aerobic b. Anaerobic c. Resistance d. Flexibility 2. Environmental a. Heat b.Cold c. Altitude d.Recreational diving (scuba) 3. Muscle a. Contraction b. Lactate kinetics c. Delayed onset muscle soreness d. Fiber types 4. Neuroendocrine 5. Respiratory 6. Circulatory 7. Special populations a. Children b. Elderly c. Athletes with chronic disease d. Disabled athletes B. Anatomy 1. Head/Neck a.Bone b. Soft tissue c. Innervation d. Vascular 2. Chest/Abdomen a.Bone b. Soft tissue c. Innervation d. Vascular 3. Back a.Bone b. Soft tissue c. Innervation 1 d. Vascular 4. Shoulder/Upper arm a. Bone b. Soft tissue c. Innervation d. Vascular 5. Elbow/Forearm a. Bone b. Soft tissue c. Innervation d. Vascular 6. Hand/Wrist a. Bone b. Soft tissue c. Innervation d. Vascular 7. Hip/Pelvis/Thigh a. Bone b. Soft tissue c. Innervation d. Vascular 8. Knee a. Bone b. Soft tissue c. Innervation d. Vascular 9. Lower Leg/Foot/Ankle a. Bone b. Soft tissue c. Innervation d. Vascular 10. Immature Skeleton a. Physes b. Apophyses C. Biomechanics 1. Throwing/Overhead activities 2. Swimming 3. Gait/Running 4. Cycling 5. Jumping activities 6. Joint kinematics D. Pharmacology 1. Therapeutic Drugs a. Analgesics b. Antibiotics c. Antidiabetic agents d. Antihypertensives e. -

Pathology and Laboratory Medicine 1
Pathology and Laboratory Medicine 1 and hepatic pathology. The rotation consists of daily interpretation of Pathology and subspecialty biopsies, participation in subspecialty conferences, slide set study, and assigned readings. Students participate in their own Laboratory Medicine learning by setting their rotation objectives with faculty at the start of their elective and following through with a schedule of clinical, laboratory and core lecture conferences. Students will need to obtain the appropriate Pathologists play many roles in medicine, from interpreting surgical staff members' permission for the rotation as follows: dermatopathology biopsies to supervising clinical laboratory testing. It has been estimated (Garth Fraga); neuropathology (Kathy Newell); renal pathology (Timothy that 70% of all medical decisions are based on data generated by Fields); breast pathology (Fang Fan); hepatic pathology (Maura O'Neil). pathology departments. The department of Pathology and Laboratory Prerequisite: Completion of the core clinical clerkships and permission of Medicine at KUMC plays an integral role in the core curriculum and also the faculty. LEC. offers elective courses to medical students interested in learning more about laboratory practice. Students in elective rotations participate in daily teaching conferences and specimen “sign-out” at the University of Kansas Hospital. They receive hands-on exposure to pathology technical methodology in the surgical pathology suite, cytopathology and hematopathology. PAON 920. Molecular Medicine: Approaches & Ethics. 2 Hours. Molecular Medicine: Approaches Ethics is a two semester course for first year MD-PhD students taught by the Director of the MD- PhD Program, with other faculty from the basic science and clinical departments. Through lectures, small group discussion, online modules, evaluation of primary literature, and presentations/discussions with current KUMC faculty, students will be introduced to the process of scientific investigation. -

DUKE UNIVERSITY School of Medicine Pathologists' Assistant
DUKE UNIVERSITY School of Medicine Pathologists’ Assistant Program Department of Pathology Academic Programs The Department of Pathology at Duke University offers a wide array of training programs to fit individual requirements and goals. The Residency Training program is an ACGME approved program and is available as an Anatomic Pathology/Clinical Pathology combined program, a shorter Anatomic Pathology only program, or an Anatomic Pathology/Neuropathology program. Subspecialty fellowships in Cytopathology, Dermatopathology, Hematopathology, Medical Microbiology, and Neuropathology are also ACGME approved. These programs provide the highest quality of graduate medical education by drawing on the depth and breadth of faculty expertise in the Department in all aspects of anatomic and clinical pathology and the availability of a wide variety of often complex clinical cases seen at Duke University Health System. For medical students interested in a career in Pathology pre-doctoral fellowships, internships and externships are available. Research Training in Experimental pathology can be obtained through Pre- and postdoctoral fellowships of one to five years. All pre-doctoral fellows are candidates for the Ph.D. degree in pathology. The Ph.D. is optional in postdoctoral programs, which provide didactic and research training in various aspects of modern experimental pathology. A two year NAACLS accredited Pathologists’ Assistant Program leads to a Master of Health Science degree, certifies graduates to sit for the ASCP Board of Certification examination, and leads to exciting career opportunities in a variety of anatomic pathology laboratory settings. Pathologists’ assistants are analogous to physician assistants, but with highly specialized training in autopsy and surgical pathology. This profession was pioneered in the Duke Department of Pathology 50 years ago, and is one of only twelve such programs in existence today. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Acute Pain Medicine & Regional Anesthesia Course
4TH ANNUAL Carlo D. Franco, MD COURSE DESCRIPTION Chairman Regional Anesthesia ACUTE PAIN MEDICINE & The Acute Pain Medicine & Regional Anesthesia Course JHS Hospital of Cook County provides an intense, hands-on cadaver workshop for civilian Professor of Anesthesiology and Anatomy and military physician anesthesiologists seeking to add Rush University Medical Center – Chicago, IL REGIONAL ANESTHESIA advanced anesthesia techniques to his/her practice. The workshop focuses on indications, anatomical considerations Ralf E. Gebhard, MD Professor of Anesthesiology COURSE and techniques for each block. Participants will work in small Associate Professor of Orthopedics and Rehabilitation groups directly with renowned faculty during the hands- Director, Division of Regional Anesthesia and Acute Perioperative Pain Management FOR MILITARY AND CIVILIAN ANESTHESIOLOGISTS on instruction with non-embalmed cadaver specimens at multiple workstations in a state-of-the-art anatomical teaching Miller School of Medicine INTERESTED IN TRAUMA AND PERIOPERATIVE laboratory. Participants will conduct actual ultrasound guided University of Miami – Miami, FL PAIN MANAGEMENT regional techniques on the aforementioned cadavers. This Harold Gelfand, MD course was formerly the Annual Comprehensive Regional Regional Anesthesia/Acute Pain Medicine Anesthesia Workshop, held for 10 years at the Uniformed Bethesda, MD Services University of the Health Sciences (USUHS) in Bethesda, Maryland. Roy Greengrass, MD Professor of Anesthesiology COURSE OBJECTIVES Mayo Clinic – Jacksonville, FL 1. Participants will review multimodal analgesia including Justin W. Heil, MD MARCH 18-19, 2016 various opioid and non-opioid agents utilized in the setting of Regional Anesthesia/Acute Pain Medicine BALTIMORE, MARYLAND acute pain. San Diego, CA 2. Participants will be able to identify the organization and fiscal concerns of an acute pain medicine service. -

Printable Version
Clinical Pathology Laboratories Drugs of Abuse Testing Offering our clients state-of-the-art testing is part of CPL’s ongoing commitment to excellence. Effective 06/18/2018, Clinical Pathology Laboratories (CPL) will replace current drug of abuse (DAU) screening and screening with reflex confirmation profiles. The new profiles provide: • Reformulation and recombination of classes for profile testing, more relevant to current societal trends • Standardization of drug thresholds to high sensitivity cutoff values • Addition of the following analytes in specific profiles for testing: o Fentanyl o Buprenorphine o 6-Acetylmorphine o MDMA (Ecstasy) • Expansion of testing for adulterants with disqualifying comments added to specimens determined to be altered • Expansion of interpretive notes on reports regarding the method, cutoff values, and specific drugs that may or may not be detected by the screening method New Profile Name EtOH Opiates Cocaine Fentanyl Oxycodone Order Code Methadone Barbiturates Cannabinoids Phencyclidine Acetylmorphine Buprenorphine Amphetamines MDMA/Ecstasy - Benzodiazepines 6 Drug of Abuse, 8 Analytes 3305 X X X X X X X X No Confirm Drug of Abuse, 8 Analytes 3306 X X X X X X X X w/ Confirm Drug of Abuse, 9 Analytes 3316 X X X X X X X X X w/ EtOH, No Confirm Drug of Abuse, 9 Analytes 3315 X X X X X X X X X w/ EtOH, w/ Confirm Drug of Abuse, 9 Analytes 3307 X X X X X X X X X No THC or Confirm Drug of Abuse, 9 Analytes 3308 X X X X X X X X X No THC, w/ Confirm Drug of Abuse, 10 Analytes 3317 X X X X X X X X X X No Confirm -

Clinical Pathology, Immunopathology and Advanced Vaccine Technology in Bovine Theileriosis: a Review
pathogens Review Clinical Pathology, Immunopathology and Advanced Vaccine Technology in Bovine Theileriosis: A Review Onyinyechukwu Ada Agina 1,2,* , Mohd Rosly Shaari 3, Nur Mahiza Md Isa 1, Mokrish Ajat 4, Mohd Zamri-Saad 5 and Hazilawati Hamzah 1,* 1 Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang 43400, Malaysia; [email protected] 2 Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, University of Nigeria Nsukka, Nsukka 410001, Nigeria 3 Animal Science Research Centre, Malaysian Agricultural Research and Development Institute, Headquarters, Serdang 43400, Malaysia; [email protected] 4 Department of Veterinary Pre-clinical sciences, Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang 43400, Malaysia; [email protected] 5 Research Centre for Ruminant Diseases, Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang 43400, Malaysia; [email protected] * Correspondence: [email protected] (O.A.A.); [email protected] (H.H.); Tel.: +60-11-352-01215 (O.A.A.); +60-19-284-6897 (H.H.) Received: 2 May 2020; Accepted: 16 July 2020; Published: 25 August 2020 Abstract: Theileriosis is a blood piroplasmic disease that adversely affects the livestock industry, especially in tropical and sub-tropical countries. It is caused by haemoprotozoan of the Theileria genus, transmitted by hard ticks and which possesses a complex life cycle. The clinical course of the disease ranges from benign to lethal, but subclinical infections can occur depending on the infecting Theileria species. The main clinical and clinicopathological manifestations of acute disease include fever, lymphadenopathy, anorexia and severe loss of condition, conjunctivitis, and pale mucous membranes that are associated with Theileria-induced immune-mediated haemolytic anaemia and/or non-regenerative anaemia. -
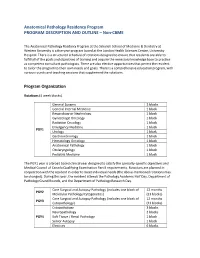
Anatomical Pathology Residency Program PROGRAM DESCRIPTION and OUTLINE – Non-CBME
Anatomical Pathology Residency Program PROGRAM DESCRIPTION AND OUTLINE – Non-CBME The Anatomical Pathology Residency Program at the Schulich School of Medicine & Dentistry at Western University is a five year program based at the London Health Sciences Centre, University Hospital. There is a structured schedule of rotations designed to ensure that residents are able to fulfill all of the goals and objectives of training and acquire the necessary knowledge base to practice as competent consultant pathologists. There are also elective opportunities that permit the resident to tailor the program to their own needs and goals. There is a comprehensive education program, with various rounds and teaching sessions that supplement the rotations. Program Organization Rotations (4 week blocks) General Surgery 2 blocks General Internal Medicine 1 block Respirology or Nephrology 1 block Gynecologic Oncology 1 block Radiation Oncology 1 block Emergency Medicine 1 block PGY1 Urology 1 block Gastroenterology 1 block Hematology Oncology 1 block Anatomical Pathology 1 block Otolaryngology 1 block Pediatric Medicine 1 block The PGY1 year is a broad based clinical year designed to satisfy the specialty-specific objectives and Medical Council of Canada Qualifying Examination Part II requirements. Rotations are planned in conjunction with the resident in order to meet individual needs (the above-mentioned rotations may be changed). During this year, the resident attends the Pathology Academic Half Day, Department of Pathology Grand Rounds, and the Department -

Overview of Pathology and Its Related Disciplines - Soheir Mahmoud Mahfouz
MEDICAL SCIENCES – Vol.I -Overview of Pathology and its Related Disciplines - Soheir Mahmoud Mahfouz OVERVIEW OF PATHOLOGY AND ITS RELATED DISCIPLINES Soheir Mahmoud Mahfouz Cairo University, Kasr El Ainy Hospital, Egypt Keywords: Pathology, Pathology disciplines, Pathology techniques, Ancillary diagnostic methods, General Pathology, Special Pathology Contents 1. Introduction 1.1 Pathology coverage 1.1.1 Etiology and Pathogenesis of a Disease 1.1.2 Manifestations of Disease (Lesions) 1.1.3 Phases Of A Disease Process (Course) 1.2 Physician’s approach to patient 1.3 Types of pathologists and affiliated specialties 1.4 Role of pathologist 2. Pathology and its related disciplines 2.1 Cytology 2.1.1 Cytology Samples 2.1.2 Technical Aspects 2.1.3 Examination of Sample and Diagnosis 3. Pathology techniques and ancillary diagnostic methods 3.1 Macroscopic pathology 3.2 Light Microscopy 3.3 Polarizing light microscopy 3.4 Electron microscopy (EM) 3.5 Confocal Microscopy 3.6 Frozen section 3.7 Cyto/histochemistry 3.8 Immunocyto/histochemical methods 3.9 Molecular and genetic methods of diagnosis 3.10 Quantitative methods 4. Types of tests used in pathology 4.1 DiagnosticUNESCO tests – EOLSS 4.2 Quantitative tests 4.3 Prognostic tests 5. The scope of SAMPLEpathology & its main divisions CHAPTERS 6. Conclusions Glossary Bibliography Biographical sketch Summary Pathology is the science of disease. It deals with deviations from normal body function and ©Encyclopedia of Life Support Systems (EOLSS) MEDICAL SCIENCES – Vol.I -Overview of Pathology and its Related Disciplines - Soheir Mahmoud Mahfouz structure. Many disciplines are involved in the study of disease, as it is necessary to understand the complex causes and effects of various disorders that affect the organs and body as a whole. -

American Society for Clinical Pathology to the Clinical Laboratory Improvement Advisory Committee
Statement from the American Society for Clinical Pathology to the Clinical Laboratory Improvement Advisory Committee The American Society for Clinical Pathology is pleased to provide this statement to the Clinical Laboratory Improvement Advisory Committee (CLIAC) on the roles, responsibilities and competencies of bioinformaticists. The completion of the Human Genome Project has resulted in vast sums of patient data, and bioinformaticists are increasingly being utilized by clinical laboratories to manage, process, and analyze it, especially in the rapidly expanding specialty of molecular diagnostics. Bioinformaticists, and the unique skills these individuals bring, are also helping to transform the practice of pathology and laboratory medicine by developing or/or enhancing the bioinformatics tools used to expand the ability of pathology and laboratory medicine to protect patient health. ASCP greatly appreciates CLIAC’s leadership by focusing attention on the valuable contribution these professionals are making and to improve their ability to do so. The following comments are based on comments provided by our membership during our efforts to respond to the questions posed by the CLIAC. CLIAC Discussion Questions: Question 1: Are Bioinformaticists needed in clinical and public health laboratories? If so, what are the current roles, responsibilities, and competencies of bioinformaticists in these settings? ASCP believes that bioinformaticists are a key component of high quality, full service clinical laboratories, though the roles and responsibilities of these professionals may vary significantly. Informaticists are critical to building the bioinformatics pipeline, which can include the software and database engineering, configuration of available bioinformatics software, and/or management and interfacing of LIS and other informatics systems, both internally within the laboratory (e.g.