The Log-Odd Normal Generalized Family of Distributions with Application
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

5. the Student T Distribution
Virtual Laboratories > 4. Special Distributions > 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 5. The Student t Distribution In this section we will study a distribution that has special importance in statistics. In particular, this distribution will arise in the study of a standardized version of the sample mean when the underlying distribution is normal. The Probability Density Function Suppose that Z has the standard normal distribution, V has the chi-squared distribution with n degrees of freedom, and that Z and V are independent. Let Z T= √V/n In the following exercise, you will show that T has probability density function given by −(n +1) /2 Γ((n + 1) / 2) t2 f(t)= 1 + , t∈ℝ ( n ) √n π Γ(n / 2) 1. Show that T has the given probability density function by using the following steps. n a. Show first that the conditional distribution of T given V=v is normal with mean 0 a nd variance v . b. Use (a) to find the joint probability density function of (T,V). c. Integrate the joint probability density function in (b) with respect to v to find the probability density function of T. The distribution of T is known as the Student t distribution with n degree of freedom. The distribution is well defined for any n > 0, but in practice, only positive integer values of n are of interest. This distribution was first studied by William Gosset, who published under the pseudonym Student. In addition to supplying the proof, Exercise 1 provides a good way of thinking of the t distribution: the t distribution arises when the variance of a mean 0 normal distribution is randomized in a certain way. -
![On the Computation of Multivariate Scenario Sets for the Skew-T and Generalized Hyperbolic Families Arxiv:1402.0686V1 [Math.ST]](https://docslib.b-cdn.net/cover/2984/on-the-computation-of-multivariate-scenario-sets-for-the-skew-t-and-generalized-hyperbolic-families-arxiv-1402-0686v1-math-st-292984.webp)
On the Computation of Multivariate Scenario Sets for the Skew-T and Generalized Hyperbolic Families Arxiv:1402.0686V1 [Math.ST]
On the Computation of Multivariate Scenario Sets for the Skew-t and Generalized Hyperbolic Families Emanuele Giorgi1;2, Alexander J. McNeil3;4 February 5, 2014 Abstract We examine the problem of computing multivariate scenarios sets for skewed distributions. Our interest is motivated by the potential use of such sets in the stress testing of insurance companies and banks whose solvency is dependent on changes in a set of financial risk factors. We define multivariate scenario sets based on the notion of half-space depth (HD) and also introduce the notion of expectile depth (ED) where half-spaces are defined by expectiles rather than quantiles. We then use the HD and ED functions to define convex scenario sets that generalize the concepts of quantile and expectile to higher dimensions. In the case of elliptical distributions these sets coincide with the regions encompassed by the contours of the density function. In the context of multivariate skewed distributions, the equivalence of depth contours and density contours does not hold in general. We consider two parametric families that account for skewness and heavy tails: the generalized hyperbolic and the skew- t distributions. By making use of a canonical form representation, where skewness is completely absorbed by one component, we show that the HD contours of these distributions are near-elliptical and, in the case of the skew-Cauchy distribution, we prove that the HD contours are exactly elliptical. We propose a measure of multivariate skewness as a deviation from angular symmetry and show that it can explain the quality of the elliptical approximation for the HD contours. -

Research Participants' Rights to Data Protection in the Era of Open Science
DePaul Law Review Volume 69 Issue 2 Winter 2019 Article 16 Research Participants’ Rights To Data Protection In The Era Of Open Science Tara Sklar Mabel Crescioni Follow this and additional works at: https://via.library.depaul.edu/law-review Part of the Law Commons Recommended Citation Tara Sklar & Mabel Crescioni, Research Participants’ Rights To Data Protection In The Era Of Open Science, 69 DePaul L. Rev. (2020) Available at: https://via.library.depaul.edu/law-review/vol69/iss2/16 This Article is brought to you for free and open access by the College of Law at Via Sapientiae. It has been accepted for inclusion in DePaul Law Review by an authorized editor of Via Sapientiae. For more information, please contact [email protected]. \\jciprod01\productn\D\DPL\69-2\DPL214.txt unknown Seq: 1 21-APR-20 12:15 RESEARCH PARTICIPANTS’ RIGHTS TO DATA PROTECTION IN THE ERA OF OPEN SCIENCE Tara Sklar and Mabel Crescioni* CONTENTS INTRODUCTION ................................................. 700 R I. REAL-WORLD EVIDENCE AND WEARABLES IN CLINICAL TRIALS ....................................... 703 R II. DATA PROTECTION RIGHTS ............................. 708 R A. General Data Protection Regulation ................. 709 R B. California Consumer Privacy Act ................... 710 R C. GDPR and CCPA Influence on State Data Privacy Laws ................................................ 712 R III. RESEARCH EXEMPTION AND RELATED SAFEGUARDS ... 714 R IV. RESEARCH PARTICIPANTS’ DATA AND OPEN SCIENCE . 716 R CONCLUSION ................................................... 718 R Clinical trials are increasingly using sensors embedded in wearable de- vices due to their capabilities to generate real-world data. These devices are able to continuously monitor, record, and store physiological met- rics in response to a given therapy, which is contributing to a redesign of clinical trials around the world. -

A Study of Non-Central Skew T Distributions and Their Applications in Data Analysis and Change Point Detection
A STUDY OF NON-CENTRAL SKEW T DISTRIBUTIONS AND THEIR APPLICATIONS IN DATA ANALYSIS AND CHANGE POINT DETECTION Abeer M. Hasan A Dissertation Submitted to the Graduate College of Bowling Green State University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY August 2013 Committee: Arjun K. Gupta, Co-advisor Wei Ning, Advisor Mark Earley, Graduate Faculty Representative Junfeng Shang. Copyright c August 2013 Abeer M. Hasan All rights reserved iii ABSTRACT Arjun K. Gupta, Co-advisor Wei Ning, Advisor Over the past three decades there has been a growing interest in searching for distribution families that are suitable to analyze skewed data with excess kurtosis. The search started by numerous papers on the skew normal distribution. Multivariate t distributions started to catch attention shortly after the development of the multivariate skew normal distribution. Many researchers proposed alternative methods to generalize the univariate t distribution to the multivariate case. Recently, skew t distribution started to become popular in research. Skew t distributions provide more flexibility and better ability to accommodate long-tailed data than skew normal distributions. In this dissertation, a new non-central skew t distribution is studied and its theoretical properties are explored. Applications of the proposed non-central skew t distribution in data analysis and model comparisons are studied. An extension of our distribution to the multivariate case is presented and properties of the multivariate non-central skew t distri- bution are discussed. We also discuss the distribution of quadratic forms of the non-central skew t distribution. In the last chapter, the change point problem of the non-central skew t distribution is discussed under different settings. -

Overview of Reliability Engineering
Overview of reliability engineering Eric Marsden <[email protected]> Context ▷ I have a fleet of airline engines and want to anticipate when they mayfail ▷ I am purchasing pumps for my refinery and want to understand the MTBF, lambda etc. provided by the manufacturers ▷ I want to compare different system designs to determine the impact of architecture on availability 2 / 32 Reliability engineering ▷ Reliability engineering is the discipline of ensuring that a system will function as required over a specified time period when operated and maintained in a specified manner. ▷ Reliability engineers address 3 basic questions: • When does something fail? • Why does it fail? • How can the likelihood of failure be reduced? 3 / 32 The termination of the ability of an item to perform a required function. [IEV Failure Failure 191-04-01] ▷ A failure is always related to a required function. The function is often specified together with a performance requirement (eg. “must handleup to 3 tonnes per minute”, “must respond within 0.1 seconds”). ▷ A failure occurs when the function cannot be performed or has a performance that falls outside the performance requirement. 4 / 32 The state of an item characterized by inability to perform a required function Fault Fault [IEV 191-05-01] ▷ While a failure is an event that occurs at a specific point in time, a fault is a state that will last for a shorter or longer period. ▷ When a failure occurs, the item enters the failed state. A failure may occur: • while running • while in standby • due to demand 5 / 32 Discrepancy between a computed, observed, or measured value or condition and Error Error the true, specified, or theoretically correct value or condition. -

Lochner</I> in Cyberspace: the New Economic Orthodoxy Of
Georgetown University Law Center Scholarship @ GEORGETOWN LAW 1998 Lochner in Cyberspace: The New Economic Orthodoxy of "Rights Management" Julie E. Cohen Georgetown University Law Center, [email protected] This paper can be downloaded free of charge from: https://scholarship.law.georgetown.edu/facpub/811 97 Mich. L. Rev. 462-563 (1998) This open-access article is brought to you by the Georgetown Law Library. Posted with permission of the author. Follow this and additional works at: https://scholarship.law.georgetown.edu/facpub Part of the Intellectual Property Law Commons, Internet Law Commons, and the Law and Economics Commons LOCHNER IN CYBERSPACE: THE NEW ECONOMIC ORTHODOXY OF "RIGHTS MANAGEMENT" JULIE E. COHEN * Originally published 97 Mich. L. Rev. 462 (1998). TABLE OF CONTENTS I. THE CONVERGENCE OF ECONOMIC IMPERATIVES AND NATURAL RIGHTS..........6 II. THE NEW CONCEPTUALISM.....................................................18 A. Constructing Consent ......................................................18 B. Manufacturing Scarcity.....................................................32 1. Transaction Costs and Common Resources................................33 2. Incentives and Redistribution..........................................40 III. ON MODELING INFORMATION MARKETS........................................50 A. Bargaining Power and Choice in Information Markets.............................52 1. Contested Exchange and the Power to Switch.............................52 2. Collective Action, "Rent-Seeking," and Public Choice.......................67 -

Understanding Functional Safety FIT Base Failure Rate Estimates Per IEC 62380 and SN 29500
www.ti.com Technical White Paper Understanding Functional Safety FIT Base Failure Rate Estimates per IEC 62380 and SN 29500 Bharat Rajaram, Senior Member, Technical Staff, and Director, Functional Safety, C2000™ Microcontrollers, Texas Instruments ABSTRACT Functional safety standards like International Electrotechnical Commission (IEC) 615081 and International Organization for Standardization (ISO) 262622 require that semiconductor device manufacturers address both systematic and random hardware failures. Systematic failures are managed and mitigated by following rigorous development processes. Random hardware failures must adhere to specified quantitative metrics to meet hardware safety integrity levels (SILs) or automotive SILs (ASILs). Consequently, systematic failures are excluded from the calculation of random hardware failure metrics. Table of Contents 1 Introduction.............................................................................................................................................................................2 2 Types of Faults and Quantitative Random Hardware Failure Metrics .............................................................................. 2 3 Random Failures Over a Product Lifetime and Estimation of BFR ...................................................................................3 4 BFR Estimation Techniques ................................................................................................................................................. 4 5 Siemens SN 29500 FIT model -

Chapter 8 Fundamental Sampling Distributions And
CHAPTER 8 FUNDAMENTAL SAMPLING DISTRIBUTIONS AND DATA DESCRIPTIONS 8.1 Random Sampling pling procedure, it is desirable to choose a random sample in the sense that the observations are made The basic idea of the statistical inference is that we independently and at random. are allowed to draw inferences or conclusions about a Random Sample population based on the statistics computed from the sample data so that we could infer something about Let X1;X2;:::;Xn be n independent random variables, the parameters and obtain more information about the each having the same probability distribution f (x). population. Thus we must make sure that the samples Define X1;X2;:::;Xn to be a random sample of size must be good representatives of the population and n from the population f (x) and write its joint proba- pay attention on the sampling bias and variability to bility distribution as ensure the validity of statistical inference. f (x1;x2;:::;xn) = f (x1) f (x2) f (xn): ··· 8.2 Some Important Statistics It is important to measure the center and the variabil- ity of the population. For the purpose of the inference, we study the following measures regarding to the cen- ter and the variability. 8.2.1 Location Measures of a Sample The most commonly used statistics for measuring the center of a set of data, arranged in order of mag- nitude, are the sample mean, sample median, and sample mode. Let X1;X2;:::;Xn represent n random variables. Sample Mean To calculate the average, or mean, add all values, then Bias divide by the number of individuals. -

A Tail Quantile Approximation Formula for the Student T and the Symmetric Generalized Hyperbolic Distribution
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Schlüter, Stephan; Fischer, Matthias J. Working Paper A tail quantile approximation formula for the student t and the symmetric generalized hyperbolic distribution IWQW Discussion Papers, No. 05/2009 Provided in Cooperation with: Friedrich-Alexander University Erlangen-Nuremberg, Institute for Economics Suggested Citation: Schlüter, Stephan; Fischer, Matthias J. (2009) : A tail quantile approximation formula for the student t and the symmetric generalized hyperbolic distribution, IWQW Discussion Papers, No. 05/2009, Friedrich-Alexander-Universität Erlangen-Nürnberg, Institut für Wirtschaftspolitik und Quantitative Wirtschaftsforschung (IWQW), Nürnberg This Version is available at: http://hdl.handle.net/10419/29554 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. may exercise further usage rights as specified in the indicated licence. www.econstor.eu IWQW Institut für Wirtschaftspolitik und Quantitative Wirtschaftsforschung Diskussionspapier Discussion Papers No. -

Chapter 4: Central Tendency and Dispersion
Central Tendency and Dispersion 4 In this chapter, you can learn • how the values of the cases on a single variable can be summarized using measures of central tendency and measures of dispersion; • how the central tendency can be described using statistics such as the mode, median, and mean; • how the dispersion of scores on a variable can be described using statistics such as a percent distribution, minimum, maximum, range, and standard deviation along with a few others; and • how a variable’s level of measurement determines what measures of central tendency and dispersion to use. Schooling, Politics, and Life After Death Once again, we will use some questions about 1980 GSS young adults as opportunities to explain and demonstrate the statistics introduced in the chapter: • Among the 1980 GSS young adults, are there both believers and nonbelievers in a life after death? Which is the more common view? • On a seven-attribute political party allegiance variable anchored at one end by “strong Democrat” and at the other by “strong Republican,” what was the most Democratic attribute used by any of the 1980 GSS young adults? The most Republican attribute? If we put all 1980 95 96 PART II :: DESCRIPTIVE STATISTICS GSS young adults in order from the strongest Democrat to the strongest Republican, what is the political party affiliation of the person in the middle? • What was the average number of years of schooling completed at the time of the survey by 1980 GSS young adults? Were most of these twentysomethings pretty close to the average on -

A Formulation for Continuous Mixtures of Multivariate Normal Distributions
A formulation for continuous mixtures of multivariate normal distributions Reinaldo B. Arellano-Valle Adelchi Azzalini Departamento de Estadística Dipartimento di Scienze Statistiche Pontificia Universidad Católica de Chile Università di Padova Chile Italia 31st March 2020 Abstract Several formulations have long existed in the literature in the form of continuous mixtures of normal variables where a mixing variable operates on the mean or on the variance or on both the mean and the variance of a multivariate normal variable, by changing the nature of these basic constituents from constants to random quantities. More recently, other mixture-type constructions have been introduced, where the core random component, on which the mixing operation oper- ates, is not necessarily normal. The main aim of the present work is to show that many existing constructions can be encompassed by a formulation where normal variables are mixed using two univariate random variables. For this formulation, we derive various general properties. Within the proposed framework, it is also simpler to formulate new proposals of parametric families and we provide a few such instances. At the same time, the exposition provides a review of the theme of normal mixtures. Key-words: location-scale mixtures, mixtures of normal distribution. arXiv:2003.13076v1 [math.PR] 29 Mar 2020 1 1 Continuous mixtures of normal distributions In the last few decades, a number of formulations have been put forward, in the context of distribution theory, where a multivariate normal variable represents the basic constituent but with the superpos- ition of another random component, either in the sense that the normal mean value or the variance matrix or both these components are subject to the effect of another random variable of continuous type. -
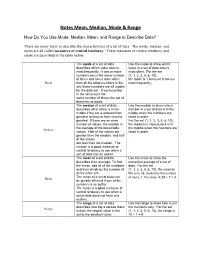
Notes Mean, Median, Mode & Range
Notes Mean, Median, Mode & Range How Do You Use Mode, Median, Mean, and Range to Describe Data? There are many ways to describe the characteristics of a set of data. The mode, median, and mean are all called measures of central tendency. These measures of central tendency and range are described in the table below. The mode of a set of data Use the mode to show which describes which value occurs value in a set of data occurs most frequently. If two or more most often. For the set numbers occur the same number {1, 1, 2, 3, 5, 6, 10}, of times and occur more often the mode is 1 because it occurs Mode than all the other numbers in the most frequently. set, those numbers are all modes for the data set. If each number in the set occurs the same number of times, the set of data has no mode. The median of a set of data Use the median to show which describes what value is in the number in a set of data is in the middle if the set is ordered from middle when the numbers are greatest to least or from least to listed in order. greatest. If there are an even For the set {1, 1, 2, 3, 5, 6, 10}, number of values, the median is the median is 3 because it is in the average of the two middle the middle when the numbers are Median values. Half of the values are listed in order. greater than the median, and half of the values are less than the median.