Robertson Basalt Tall Open Forest in the Sydney Basin Bioregion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NZDFI Wood Quality Research Plan
Section 2: NZDFI Wood Quality Research Plan 1 Background NZDFI aims to establish a new hardwood forest industry based on naturally durable eucalypts. NZDFI has identified sustainably-grown naturally durable posts and poles for the agricultural industry as key products as an alternative to CCA treated pine (Millen, 2009). For these products natural durability is essential. The timber of these eucalypt species also has a high Modulus of Elasticity (MoE). A second targeted product is high stiffness LVL. These products can be produced from short- rotation smaller diameter trees. As these timbers also have attractively coloured heartwood, high quality appearance grade solid timber products are also a possibility if trees are grown on a longer rotation to a larger size. To ensure a quality product the variability in these properties can be reduced by genetic selection. Furthermore the trees must be easy to process, which requires trees with low growth-stresses and a low level of drying defects. NZDFI’s wood quality research programme addresses these problems to facilitate a viable hardwood forest industry based on naturally durable eucalypts. Some research work is financed through SWP (MBIE) while other work is funded through SFF (MPI) and other sources. Wood quality is a key research theme alongside other essential areas such as tree health, tree growth, propagation and forest management. 2 Natural durability Wood is a bio-material and biodegradable. Biodegradability is a positive attribute when considering the disposal at the end of a product’s life. However, susceptibility of wood to decay by organisms can result in premature product failure. -

Wednesday Walk in Monga Forest – Loop Off Reidsdale Road – 4 March 2009
Wednesday Walk in Monga Forest – Loop off Reidsdale Road – 4 March 2009 We left cars at both ends of this loop (shown on the 1:25,000 Monga map) which is on the southern side of Reidsdale Road. We started at the top, ie the western end, and were in tall forest with a moist understorey of an interesting array of plants including many tree ferns (Dicksonia antarctica and Cyathea australis), Goodenia ovata, Persoonia linearis, Platysace lanceolata, Coprosma quadrifida, Leucopogon lanceolatus. The first part of the track was easy to follow but the second part was overgrown and covered in debris – easily missed. Image by Jean Geue Image by Jean Geue Loop track Image by Jean Geue Persoonia linearis Image by Roger Farrow Polyscias sambucifolia fruit Image by Roger Farrow Smilax australis fruit Image by Roger Farrow Xerochrysum bracteatum Image by Jean Geue Platysace lanceolata Image by Jean Geue Prepared by the Wednesday Walkers of the Australian Native Plants Society, Canberra Region Plant List for Reidsdale Road Loop – Monga Forest – 4 March 2009 ? indicates that those present were unsure of the plant name Acacia ? falcata Lepidosperma laterale Acacia falciformis Leucopogon lanceolatus Acacia obtusifolia Lomandra longifolia Acacia trachyphloia Lycopodium deuterodensum Acaena novae-zelandiae Opercularia hispida Adiantum aethiopicum Oxalis sp. Amperea xiphoclada Ozothamnus ? argophyllus Arrhenechthites mixta Persoonia linearis Bedfordia arborescens Pimelea ligustrina Billardiera scandens Plantago debilis Blechnum nudum Platysace lanceolata Bursaria spinosa Poa meionectes Carex appressa Polyscias sambucifolia (fern leaf) Carex inversa Pomaderris aspera Cassinia aculeata Pomaderris elliptica Cassinia longifolia Poranthera microphylla Clematis aristata Pratia purpurascens Coprosma quadrifida Prostanthera lasianthos Coronidium scorpioides Prunella vulgaris Cotula alpina Pteridium esculentum Daviesia ulicifolia Pterostylis sp. -

Solasodine Production from Solanum Laciniatum in the South Island of New Zealand
SOLASODINE PRODUCTION FROM SOLANUM LACINIATUM IN THE SOUTH ISLAND OF NEW ZEALAND D. J. G. Davies and J. D. Mann Crop Research Division and Applied Biochemistry Division DSIR, Lincoln, Canterbury ABSTRACT This paper reviews agronomic studies on the production of solasodine-containing leaves from Solanum laciniatum grown as an annual crop in the South Island. The best yields were below 200 kg ha-I of solasodine, and well below commercial feasibility. INTRODUCTION Solasodine, a steroidal alkaloid valuable to the irrigations that are necessary' stimulate weed growth. pharmaceutical industry, can be extracted from the Trifluralin (2 1 ha- 1 ) was routinely incorporated by leaves of Solanum aviculare and S. laciniatum, as well discing before direct sowing, but a pre-emergence as from the fruits of these and numerous other follow-up spray with paraquat or diquat was also species of Solanum. The feasibility of using leaves for found to be necessary. Timing the latter spray was commercial extraction from S. laciniatum, on an difficult because of the prolonged germination period annual basis, is uncertain. of untreated Solanum seed. For transplants, This paper summarises four years of research on metribuzin (0.5 kg ha-1 ) applied before planting gave the production of solasodine from S. laciniatum satisfactory weed control (Betts, 1975). (poroporo) in the South Island of New Zealand. The problems of establishment (including planting time, Climatic requirements method, and density), fertilisation, harvt;sting and S. laciniatum grows well in coastal regions that are drying are covered; details of the extraction and relatively frostfree and have a uniformly distributed chemical modification of solasodine are being rainfall. -

Contents About This Booklet 2 1
Contents About this booklet 2 1. Why indigenous gardening? 3 Top ten reasons to use indigenous plants 3 Indigenous plants of Whitehorse 4 Where can I buy indigenous plants of Whitehorse? 4 2. Sustainable Gardening Principles 5 Make your garden a wildlife garden 6 3. Tips for Successful Planting 8 1. Plant selection 8 2. Pre-planting preparation 10 3. Planting technique 12 4. Early maintenance 14 4. Designing your Garden 16 Climbers 16 Hedges and borders 17 Groundcovers and fillers 17 Lawn alternatives 18 Feature trees 18 Screen plants 19 Damp & shady spots 19 Edible plants 20 Colourful flowers 21 5. 94 Species Indigenous to Whitehorse 23 6. Weeds of Whitehorse 72 7. Further Resources 81 8. Index of Plants 83 Alphabetically by Botanical Name 83 Alphabetically by Common Name 85 9. Glossary 87 1 In the spirit of About this booklet reconciliation, Whitehorse City Council This booklet has been written by Whitehorse acknowledges the City Council to help gardeners and landscapers Wurundjeri people as adopt sustainable gardening principles by using the traditional owners indigenous plants commonly found in Whitehorse. of the land now known The collective effort of residents gardening with as Whitehorse and pays indigenous species can make a big difference to respects to its elders preserving and enhancing our biodiversity. past and present. We would like to acknowledge the volunteers of the Blackburn & District Tree Preservation Society, Whitehorse Community Indigenous Plant Project Inc. (Bungalook Nursery) and Greenlink Box Hill Nursery for their efforts to protect and enhance the indigenous flora of Whitehorse. Information provided by these groups is included in this guide. -
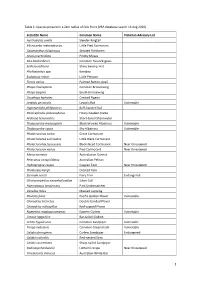
Scientific Name Common Name Victorian A
Table 1: Species present in a 2km radius of Crib Point (VBA database search 13 Aug 2020) Scientific Name Common Name Victorian Advisory List Austrolestes analis Slender Ringtail Microcarbo melanoleucos Little Pied Cormorant Calamanthus fuliginosus Striated Fieldwren Acacia verticillata Prickly Moses Poa labillardierei Common Tussock-grass Selliera radicans Shiny Swamp-mat Phyllostachys spp. Bamboo Eudyptula minor Little Penguin Turnix varius Painted Button-quail Phaps chalcoptera Common Bronzewing Phaps elegans Brush Bronzewing Ocyphaps lophotes Crested Pigeon Lewinia pectoralis Lewin's Rail Vulnerable Hypotaenidia philippensis Buff-banded Rail Poliocephalus poliocephalus Hoary-headed Grebe Ardenna tenuirostris Short-tailed Shearwater Thalassarche melanophris Black-browed Albatross Vulnerable Thalassarche cauta Shy Albatross Vulnerable Phalacrocorax carbo Great Cormorant Phalacrocorax sulcirostris Little Black Cormorant Phalacrocorax fuscescens Black-faced Cormorant Near threatened Phalacrocorax varius Pied Cormorant Near threatened Morus serrator Australasian Gannet Pelecanus conspicillatus Australian Pelican Hydroprogne caspia Caspian Tern Near threatened Thalasseus bergii Crested Tern Sternula nereis Fairy Tern Endangered Chroicocephalus novaehollandiae Silver Gull Haematopus longirostris Pied Oystercatcher Vanellus miles Masked Lapwing Pluvialis fulva Pacific Golden Plover Vulnerable Charadrius bicinctus Double-banded Plover Charadrius ruficapillus Red-capped Plover Numenius madagascariensis Eastern Curlew Vulnerable Limosa lapponica -

Oemona Hirta
EPPO Datasheet: Oemona hirta Last updated: 2021-07-29 IDENTITY Preferred name: Oemona hirta Authority: (Fabricius) Taxonomic position: Animalia: Arthropoda: Hexapoda: Insecta: Coleoptera: Cerambycidae Other scientific names: Isodera villosa (Fabricius), Oemona humilis Newman, Oemona villosa (Fabricius), Saperda hirta Fabricius, Saperda villosa Fabricius Common names: lemon tree borer view more common names online... EPPO Categorization: A1 list more photos... view more categorizations online... EU Categorization: A1 Quarantine pest (Annex II A) EPPO Code: OEMOHI Notes on taxonomy and nomenclature Lu & Wang (2005) revised the genus Oemona, which has 4 species: O. hirta, O. plicicollis, O. separata and O. simplicicollis. They provided an identification key to species and detailed descriptions. They also performed a phylogenetic analysis of all species, suggesting that O. hirta and O. plicicollis are sister species and most similar morphologically. HOSTS O. hirta is a highly polyphagous longhorn beetle. Its larvae feed on over 200 species of trees and shrubs from 63 (Lu & Wang, 2005; Wang, 2017) to 81 (EPPO, 2014) families. Its original hosts were native New Zealand plants, but it expanded its host range to many species exotic to New Zealand, ranging from major fruit, nut, forest and ornamental trees to shrubs and grapevines. Host list: Acacia dealbata, Acacia decurrens, Acacia floribunda, Acacia longifolia, Acacia melanoxylon, Acacia pycnantha, Acer pseudoplatanus, Acer sp., Aesculus hippocastanum, Agathis australis, Albizia julibrissin, Alectryon excelsus, Alnus glutinosa, Alnus incana, Aristotelia serrata, Asparagus setaceus, Avicennia marina, Avicennia resinifera, Azara sp., Betula nigra, Betula pendula, Betula sp., Brachyglottis greyi, Brachyglottis repanda, Brachyglottis rotundifolia, Buddleia davidii, Camellia sp., Carmichaelia australis, Casimiroa edulis, Cassinia leptophylla, Cassinia retorta, Castanea sativa, Casuarina cunninghamiana, Casuarina sp., Celtis australis, Cestrum elegans, Chamaecyparis sp., Chamaecytisus prolifer subsp. -

Post-Fire Recovery of Woody Plants in the New England Tableland Bioregion
Post-fire recovery of woody plants in the New England Tableland Bioregion Peter J. ClarkeA, Kirsten J. E. Knox, Monica L. Campbell and Lachlan M. Copeland Botany, School of Environmental and Rural Sciences, University of New England, Armidale, NSW 2351, AUSTRALIA. ACorresponding author; email: [email protected] Abstract: The resprouting response of plant species to fire is a key life history trait that has profound effects on post-fire population dynamics and community composition. This study documents the post-fire response (resprouting and maturation times) of woody species in six contrasting formations in the New England Tableland Bioregion of eastern Australia. Rainforest had the highest proportion of resprouting woody taxa and rocky outcrops had the lowest. Surprisingly, no significant difference in the median maturation length was found among habitats, but the communities varied in the range of maturation times. Within these communities, seedlings of species killed by fire, mature faster than seedlings of species that resprout. The slowest maturing species were those that have canopy held seed banks and were killed by fire, and these were used as indicator species to examine fire immaturity risk. Finally, we examine whether current fire management immaturity thresholds appear to be appropriate for these communities and find they need to be amended. Cunninghamia (2009) 11(2): 221–239 Introduction Maturation times of new recruits for those plants killed by fire is also a critical biological variable in the context of fire Fire is a pervasive ecological factor that influences the regimes because this time sets the lower limit for fire intervals evolution, distribution and abundance of woody plants that can cause local population decline or extirpation (Keith (Whelan 1995; Bond & van Wilgen 1996; Bradstock et al. -

Oil and Fatty Acids in Seed of Eggplant (Solanum Melongena
American Journal of Agricultural and Biological Sciences Original Research Paper Oil and Fatty Acids in Seed of Eggplant ( Solanum melongena L.) and Some Related and Unrelated Solanum Species 1Robert Jarret, 2Irvin Levy, 3Thomas Potter and 4Steven Cermak 1Department of Agriculture, Agricultural Research Service, Plant Genetic Resources Unit, 1109 Experiment Street, Griffin, Georgia 30223, USA 2Department of Chemistry, Gordon College, 255 Grapevine Road, Wenham, MA 01984 USA 3Department of Agriculture, Agricultural Research Service, Southeast Watershed Research Laboratory, P.O. Box 748, Tifton, Georgia 31793 USA 4Department of Agriculture, Agricultural Research Service, Bio-Oils Research Unit, 1815 N. University St., Peoria, IL 61604 USA Article history Abstract: The seed oil content of 305 genebank accessions of eggplant Received: 30-09-2015 (Solanum melongena ), five related species ( S. aethiopicum L., S. incanum Revised: 30-11-2015 L., S. anguivi Lam., S. linnaeanum Hepper and P.M.L. Jaeger and S. Accepted: 03-05-2016 macrocarpon L.) and 27 additional Solanum s pecies, was determined by NMR. Eggplant ( S. melongena ) seed oil content varied from 17.2% (PI Corresponding Author: Robert Jarret 63911317471) to 28.0% (GRIF 13962) with a mean of 23.7% (std. dev = Department of Agriculture, 2.1) across the 305 samples. Seed oil content in other Solanum species Agricultural Research Service, varied from 11.8% ( S. capsicoides-PI 370043) to 44.9% ( S. aviculare -PI Plant Genetic Resources Unit, 420414). Fatty acids were also determined by HPLC in genebank 1109 Experiment Street, accessions of S. melongena (55), S. aethiopicum (10), S. anguivi (4), S. Griffin, Georgia 30223, USA incanum (4) and S. -

A Molecular Phylogeny of the Solanaceae
TAXON 57 (4) • November 2008: 1159–1181 Olmstead & al. • Molecular phylogeny of Solanaceae MOLECULAR PHYLOGENETICS A molecular phylogeny of the Solanaceae Richard G. Olmstead1*, Lynn Bohs2, Hala Abdel Migid1,3, Eugenio Santiago-Valentin1,4, Vicente F. Garcia1,5 & Sarah M. Collier1,6 1 Department of Biology, University of Washington, Seattle, Washington 98195, U.S.A. *olmstead@ u.washington.edu (author for correspondence) 2 Department of Biology, University of Utah, Salt Lake City, Utah 84112, U.S.A. 3 Present address: Botany Department, Faculty of Science, Mansoura University, Mansoura, Egypt 4 Present address: Jardin Botanico de Puerto Rico, Universidad de Puerto Rico, Apartado Postal 364984, San Juan 00936, Puerto Rico 5 Present address: Department of Integrative Biology, 3060 Valley Life Sciences Building, University of California, Berkeley, California 94720, U.S.A. 6 Present address: Department of Plant Breeding and Genetics, Cornell University, Ithaca, New York 14853, U.S.A. A phylogeny of Solanaceae is presented based on the chloroplast DNA regions ndhF and trnLF. With 89 genera and 190 species included, this represents a nearly comprehensive genus-level sampling and provides a framework phylogeny for the entire family that helps integrate many previously-published phylogenetic studies within So- lanaceae. The four genera comprising the family Goetzeaceae and the monotypic families Duckeodendraceae, Nolanaceae, and Sclerophylaceae, often recognized in traditional classifications, are shown to be included in Solanaceae. The current results corroborate previous studies that identify a monophyletic subfamily Solanoideae and the more inclusive “x = 12” clade, which includes Nicotiana and the Australian tribe Anthocercideae. These results also provide greater resolution among lineages within Solanoideae, confirming Jaltomata as sister to Solanum and identifying a clade comprised primarily of tribes Capsiceae (Capsicum and Lycianthes) and Physaleae. -

List of Plants
Indigenous Plant Nursery Plant Species List The following plant list contains some of the local native plants that may be available from the Edendale Indigenous Plant Nursery. Availability can vary so please contact the nursery for specific and seasonal availability of plants. Contact details: [email protected] Phone (03) 9433 3703 30 Gastons Road, Eltham VIC 3091 Open 7 days per week, 9.30am to 4.30pm Trees Species Common Name Size (height x width) Acacia dealbata Silver Wattle 6 – 30m x 5 – 10m Acacia implexa Lightwood 5 – 15m x 4 – 7m Acacia pycnantha Golden Wattle 3 – 10m x 2 – 5m Acacia mearnsii Black Wattle 8 – 25m x 6 – 10m Acacia melanoxylon Blackwood 5 – 30m x 4 – 15m Allocasuarina littoralis Black Sheoke 4 – 8m x 2 – 5m Allocasuarina verticillata Drooping Sheoke 4 – 11m x 3 – 6m Banksia marginata Silver Banksia 1 – 10m x 1 – 5m Callitris rhomboidea Oyster Bay Pine 9 – 15 m high Eucalyptus blakelyi Blakely’s Red Gum 15 – 24m x 10 – 15m Eucalyptus camaldulensis River Red Gum 15 – 50m x 15 – 35m Eucalyptus goniocalyx Long-leaved Box 8 – 20m x 6 – 15m Eucalyptus leucoxylon Yellow Gum 10 – 20m x 6 – 20m Eucalyptus macrorhyncha Red Stringybark 10 – 35m x 10 – 20m Eucalyptus melliodora Yellow Box 10 – 30m x 8 – 25m Eucalyptus ovata Swamp Gum 8 – 30m x 8 – 20m Eucalyptus pauciflora Snow Gum 8 – 12m x 6 – 10m Eucalyptus polyanthemos Red Box 7 – 25m x 5 – 15m Eucalyptus radiata Narrow-leaved Peppermint 10 – 30m x 6 – 20m Eucalyptus rubida Candlebark Gum 10 – 25m x 10 – 20m Eucalyptus tricarpa Red Ironbark 10 – 30m x -

2016 Census of the Vascular Plants of Tasmania
A CENSUS OF THE VASCULAR PLANTS OF TASMANIA, INCLUDING MACQUARIE ISLAND MF de Salas & ML Baker 2016 edition Tasmanian Herbarium, Tasmanian Museum and Art Gallery Department of State Growth Tasmanian Vascular Plant Census 2016 A Census of the Vascular Plants of Tasmania, Including Macquarie Island. 2016 edition MF de Salas and ML Baker Postal address: Street address: Tasmanian Herbarium College Road PO Box 5058 Sandy Bay, Tasmania 7005 UTAS LPO Australia Sandy Bay, Tasmania 7005 Australia © Tasmanian Herbarium, Tasmanian Museum and Art Gallery Published by the Tasmanian Herbarium, Tasmanian Museum and Art Gallery GPO Box 1164 Hobart, Tasmania 7001 Australia www.tmag.tas.gov.au Cite as: de Salas, M.F. and Baker, M.L. (2016) A Census of the Vascular Plants of Tasmania, Including Macquarie Island. (Tasmanian Herbarium, Tasmanian Museum and Art Gallery. Hobart) www.tmag.tas.gov.au ISBN 978-1-921599-83-5 (PDF) 2 Tasmanian Vascular Plant Census 2016 Introduction The classification systems used in this Census largely follow Cronquist (1981) for flowering plants (Angiosperms) and McCarthy (1998) for conifers, ferns and their allies. The same classification systems are used to arrange the botanical collections of the Tasmanian Herbarium and by the Flora of Australia series published by the Australian Biological Resources Study (ABRS). For a more up-to-date classification of the flora refer to The Flora of Tasmania Online (Duretto 2009+) which currently follows APG II (2003). This census also serves as an index to The Student’s Flora of Tasmania (Curtis 1963, 1967, 1979; Curtis & Morris 1975, 1994). Species accounts can be found in The Student’s Flora of Tasmania by referring to the volume and page number reference that is given in the rightmost column (e.g. -

07 Shelbourne
256 New Zealand Journal of Forestry Science 32(2) PERFORMANCE TO AGE 22 YEARS OF 49 EUCALYPTS IN THE WAIRARAPA DISTRICT, NEW ZEALAND, AND REVIEW OF RESULTS FROM OTHER TRIALS C. J. A. SHELBOURNE, New Zealand Forest Research Institute, Private Bag 3020, Rotorua, New Zealand B. T. BULLOCH, P. O. Box 7097, Palmerston North, New Zealand C. B. LOW and R. M. McCONNOCHIE New Zealand Forest Research Institute, Private Bag 3020, Rotorua, New Zealand (Received for publication 30 April 2002; revision 24 September 2002) ABSTRACT Trials of 49 eucalypt species were established in 1979 in the Wairarapa district at Kahuiti and Pakaraka, originally to test species for their potential to stabilise erodable land for pastoral use. Trials were planted in a randomised complete block design with five replications of four-tree row plots of each seedlot (paired rows of four trees of species with only a single seedlot). The species included Corymbia maculata (Hook.)K.D.Hill & L.A.S.Johnson, E. cladocalyx F.Muell., four stringybarks (including E. muelleriana A.W.Howitt and E. globoidea Blakely), nine ashes (including E. fastigata Deane & Maiden, E. regnans F.Muell., and E. obliqua L’Herit), seven peppermints, and 18 gums (including E. nitens (Deane & Maiden) Maiden). Because of heavy thinning at Pakaraka, the Kahuiti trial only was assessed at age 22 years on production forestry criteria — diameter at breast height (dbh), stem straightness, malformation, crown health, and number of potential 5-m sawlogs per tree The 12 best-grown species for mean tree dbh at Kahuiti, were ranked: E. globoidea, E. muelleriana (stringybarks), E.