(12) Patent Application Publication (10) Pub. No.: US 2015/0150887 A1 COELINGHBENNINK Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
(12) United States Patent (10) Patent No.: US 7,871,995 B2 Bunschoten Et Al
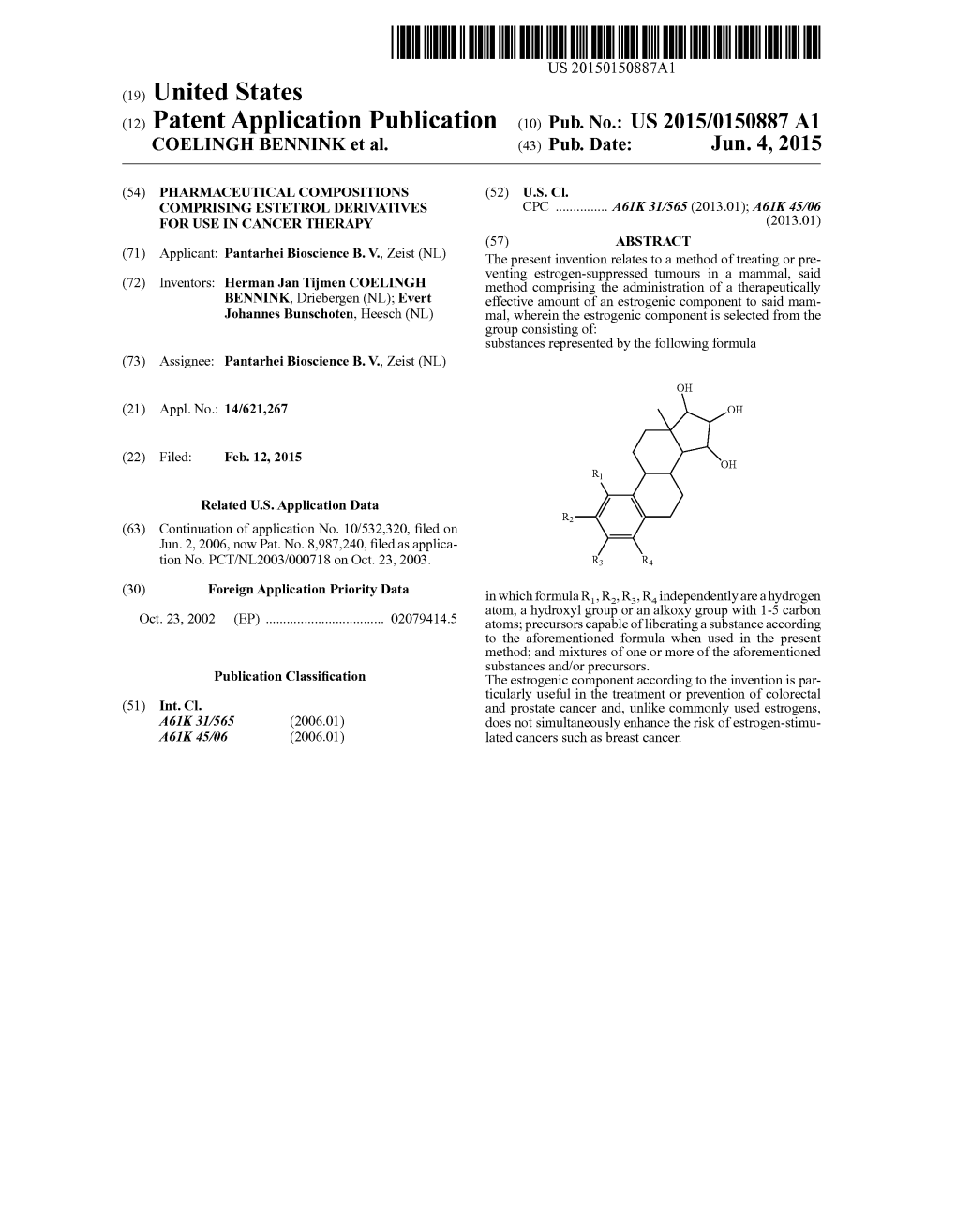
US00787 1995 B2 (12) United States Patent (10) Patent No.: US 7,871,995 B2 Bunschoten et al. (45) Date of Patent: *Jan. 18, 2011 (54) DRUG DELIVERY SYSTEM COMPRISINGA (52) U.S. Cl. ....................................... 514/171; 514/182 TETRAHYDROXYLATED ESTROGEN FOR (58) Field of Classification Search ................. 514/171, USE IN HORMONAL CONTRACEPTION 514f182 See application file for complete search history. (75) Inventors: Evert Johannes Bunschoten, Heesch (NL); Herman Jan Tijmen Coelingh (56) References Cited Bennink, Driebergen (NL); Christian U.S. PATENT DOCUMENTS Franz Holinka, New York, NY (US) 3,440,320 A 4, 1969 Sackler et al. O O 3,797.494. A 3, 1974 Zaffaroni (73) Assignee: Pantarhei Bioscience B.V., Al Zeist 4,460.372 A 7/1984 Campbell et al. (NL) 4,573.996 A 3/1986 Kwiatek et al. 4,624,665 A 1 1/1986 Nuwayser (*) Notice: Subject to any disclaimer, the term of this 4,722,941 A 2f1988 Eckert et al. patent is extended or adjusted under 35 4,762,717 A 8/1988 Crowley, Jr. U.S.C. 154(b) by 1233 days. 4.937,238 A 6, 1990 Lemon 5,063,507 A 1 1/1991 Lindsey et al. This patent is Subject to a terminal dis- 5,130,137 A 7/1992 Crowley, Jr. claimer. 5,211,952 A 5/1993 Spicer et al. 5,223,261 A 6/1993 Nelson et al. 5,340,584 A 8/1994 Spicer et al. (21) Appl. No.: 10/478,357 5,340,585 A 8/1994 Pike et al. 1-1. 5,340,586 A 8, 1994 Pike et al. -

Androgen Excess in Breast Cancer Development: Implications for Prevention and Treatment
26 2 Endocrine-Related G Secreto et al. Androgen excess in breast 26:2 R81–R94 Cancer cancer development REVIEW Androgen excess in breast cancer development: implications for prevention and treatment Giorgio Secreto1, Alessandro Girombelli2 and Vittorio Krogh1 1Epidemiology and Prevention Unit, Fondazione IRCCS – Istituto Nazionale dei Tumori, Milano, Italy 2Anesthesia and Critical Care Medicine, ASST – Grande Ospedale Metropolitano Niguarda, Milano, Italy Correspondence should be addressed to G Secreto: [email protected] Abstract The aim of this review is to highlight the pivotal role of androgen excess in the Key Words development of breast cancer. Available evidence suggests that testosterone f breast cancer controls breast epithelial growth through a balanced interaction between its two f ER-positive active metabolites: cell proliferation is promoted by estradiol while it is inhibited by f ER-negative dihydrotestosterone. A chronic overproduction of testosterone (e.g. ovarian stromal f androgen/estrogen balance hyperplasia) results in an increased estrogen production and cell proliferation that f androgen excess are no longer counterbalanced by dihydrotestosterone. This shift in the androgen/ f testosterone estrogen balance partakes in the genesis of ER-positive tumors. The mammary gland f estradiol is a modified apocrine gland, a fact rarely considered in breast carcinogenesis. When f dihydrotestosterone stimulated by androgens, apocrine cells synthesize epidermal growth factor (EGF) that triggers the ErbB family receptors. These include the EGF receptor and the human epithelial growth factor 2, both well known for stimulating cellular proliferation. As a result, an excessive production of androgens is capable of directly stimulating growth in apocrine and apocrine-like tumors, a subset of ER-negative/AR-positive tumors. -

Classification and Pharmacology of Progestins
Maturitas 46S1 (2003) S7–S16 Classification and pharmacology of progestins Adolf E. Schindler a,∗, Carlo Campagnoli b, René Druckmann c, Johannes Huber d, Jorge R. Pasqualini e, Karl W. Schweppe f, Jos H. H. Thijssen g a Institut für Medizinische Forschung und Fortbildung, Universitätsklinikum, Hufelandstr. 55, Essen 45147, Germany b Ospedale Ginecologico St. Anna, Corso Spezia 60, 10126 Torino, Italy c Ameno-Menopause-Center, 12, Rue de France, 06000 Nice, France d Abt. für Gynäkologische Endokrinologie, AKH Wien, Währingergürtel 18-20, 1090 Wien, Austria e Institute de Puériculture26, Boulevard Brune, 75014 Paris, France f Abt. für Gynäkologie und Geburtshilfe, Ammerland Klinik, Langestr.38, 26622 Westerstede, Germany g Department of Endocrinology, Universitair Medisch Centrum Utrecht, P.O. Box 85090, 3508 AB Utrecht, The Netherlands Abstract Besides the natural progestin, progesterone, there are different classes of progestins, such as retroprogesterone (i.e. dydroges- terone), progesterone derivatives (i.e. medrogestone) 17␣-hydroxyprogesterone derivatives (i.e. chlormadinone acetate, cypro- terone acetate, medroxyprogesterone acetate, megestrol acetate), 19-norprogesterone derivatives (i.e. nomegestrol, promege- stone, trimegestone, nesterone), 19-nortestosterone derivatives norethisterone (NET), lynestrenol, levonorgestrel, desogestrel, gestodene, norgestimate, dienogest) and spironolactone derivatives (i.e. drospirenone). Some of the synthetic progestins are prodrugs, which need to be metabolized to become active compounds. Besides -

Study Protocol: LPCN 1021-18-001
Oral Testosterone Undecanoate Protocol No. LPCN 1021-18-001 Lipocine Inc. TU, LPCN 1021 675 Arapeen Drive, Suite 202 Salt Lake City, UT-84108 1.0 TITLE PAGE Clinical Study Protocol: LPCN 1021-18-001 Ambulatory Blood Pressure Monitoring in Oral Testosterone Undecanoate (TU, LPCN 1021) Treated Hypogonadal Men. Investigational Product : Testosterone Undecanoate (TU, LPCN 1021) Date of Protocol : 19 February 2019 FDA IND No. : 106476 Development Phase : Phase 3 Testosterone replacement therapy in adult, 18 years or older, males for conditions associated with a deficiency or absence of endogenous Indication : testosterone – primary hypogonadism (congenital or acquired) or secondary hypogonadism (congenital or acquired) Investigator : Multi-Center, US Sponsor : Lipocine Inc. 675 Arapeen Drive, Suite 202, Salt Lake City, Utah – 84108 Tel: +1-801-994-7383 Fax: +1-801-994-7388 Sponsor / : Nachiappan Chidambaram Emergency Contact 675 Arapeen Drive, Suite 202, Salt Lake City, Utah – 84108 Tel: +1-801-994-7383, Ext 2188 Fax: +1-801-994-7388 Email: [email protected] Protocol Version : 06 Confidentiality Statement This document is a confidential communication of Lipocine Inc. Acceptance of this document signifies agreement by the recipient that no unpublished information contained within will be published or disclosed to a third party without prior written approval, except that this document may be disclosed to an Institutional Review Board under the same conditions of confidentiality. Date: 19 February 2019 Confidential Page 1 of 50 Oral Testosterone Undecanoate Protocol No. LPCN 1021-18-001 Lipocine Inc. TU, LPCN 1021 675 Arapeen Drive, Suite 202 Salt Lake City, UT-84108 2.0 SUMMARY OF CHANGES TO PROTOCOL VERSION 2 Version 02 of the LPCN 1021-18-001 study protocol was developed to make the following changes to the study: • Added sexual desire and sexual distress questions to pre-treatment and post-treatment phases of the study. -

Estrogen Deficiency During Menopause and Its Management: a Current Update V
Review Article Estrogen deficiency during menopause and its management: A current update V. T. Hemalatha1, A. Julius2, S. P. Kishore Kumar3, L. Vijayalakshmi4, Shankar Narayanan1 ABSTRACT Different phases of a woman’s life: Puberty, menses, pregnancy, and menopause have varied influence on her oral health. During the menopause, women go through biological and endocrine changes, particularly in their sex steroid hormone production, affecting their health. Sex hormones strongly influence body fat distribution and adipocyte differentiation. Estrogens and testosterone differentially affect adipocyte physiology, but the importance of estrogens in the development of metabolic diseases during menopause is disputed. Estrogens and estrogens receptor regulate various aspects of glucose and lipid metabolism. Disturbances of this metabolic signal lead to the development of metabolic syndrome and a higher cardiovascular risk in woman. The absence of estrogens is a clue factor in the onset of cardiovascular disease during the menopausal period, which is characterized by lipid profile variations and predominant abdominal fat accumulation. However, influence of the absence of these hormones and its relationship to higher obesity in women during menopause is not clear. This systematic review discusses of the role of estrogens and estrogen receptors in adipocyte differentiation and its various effects and brief discussion on its management. KEY WORDS: Estrogen hormone (estrogens/progestogens) replacement therapy, Menopause, Weight gain INTRODUCTION BODY CHANGES AT MENOPAUSE Menopause occurs when a woman stops ovulating and As we age, our muscles decrease in bulk and our her monthly period (menstruation) stops. metabolism slows down. These changes cancontribute to weight gain around the time of menopause. Other As women age, into their 40s and 50s, there is a tendency physical changes associated with menopause may to gain weight. -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

The Inhaled Steroid Ciclesonide Blocks SARS-Cov-2 RNA Replication by Targeting Viral
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.22.258459; this version posted August 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting viral 2 replication-transcription complex in culture cells 3 4 Shutoku Matsuyamaa#, Miyuki Kawasea, Naganori Naoa, Kazuya Shiratoa, Makoto Ujikeb, Wataru 5 Kamitanic, Masayuki Shimojimad, and Shuetsu Fukushid 6 7 aDepartment of Virology III, National Institute of Infectious Diseases, Tokyo, Japan 8 bFaculty of Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo, Japan 9 cDepartment of Infectious Diseases and Host Defense, Gunma University Graduate School of 10 Medicine, Gunma, Japan 11 dDepartment of Virology I, National Institute of Infectious Diseases, Tokyo, Japan. 12 13 Running Head: Ciclesonide blocks SARS-CoV-2 replication 14 15 #Address correspondence to Shutoku Matsuyama, [email protected] 16 17 Word count: Abstract 149, Text 3,016 bioRxiv preprint doi: https://doi.org/10.1101/2020.08.22.258459; this version posted August 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 18 Abstract 19 We screened steroid compounds to obtain a drug expected to block host inflammatory responses and 20 MERS-CoV replication. Ciclesonide, an inhaled corticosteroid, suppressed replication of MERS-CoV 21 and other coronaviruses, including SARS-CoV-2, the cause of COVID-19, in cultured cells. The 22 effective concentration (EC90) of ciclesonide for SARS-CoV-2 in differentiated human bronchial 23 tracheal epithelial cells was 0.55 μM. -

Progestogens and Venous Thromboembolism Among Postmenopausal Women Using Hormone Therapy. Marianne Canonico, Geneviève Plu-Bureau, Pierre-Yves Scarabin
Progestogens and venous thromboembolism among postmenopausal women using hormone therapy. Marianne Canonico, Geneviève Plu-Bureau, Pierre-Yves Scarabin To cite this version: Marianne Canonico, Geneviève Plu-Bureau, Pierre-Yves Scarabin. Progestogens and venous throm- boembolism among postmenopausal women using hormone therapy.. Maturitas, Elsevier, 2011, 70 (4), pp.354-60. 10.1016/j.maturitas.2011.10.002. inserm-01148705 HAL Id: inserm-01148705 https://www.hal.inserm.fr/inserm-01148705 Submitted on 5 May 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Progestogens and VTE Finale version Progestogens and venous thromboembolism among postmenopausal women using hormone therapy Marianne Canonico1,2, Geneviève Plu-Bureau1,3 and Pierre-Yves Scarabin1,2 1 Centre for Research in Epidemiology and Population Health, U1018, Hormones and Cardiovascular Disease 2 University Paris-Sud, UMR-S 1018, Villejuif, France 3 University Paris Descartes and Hôtel-Dieu Hospital, Paris, France Adresse: 16 av. Paul Vaillant Couturier 94807 Villejuif Cedex Tel: +33 1 45 59 51 66 Fax: +33 1 45 59 51 70 Corresponding author: Marianne Canonico ([email protected]) 1/21 Progestogens and VTE Finale version Abstract Hormone therapy (HT) is the most effective treatment for correcting menopausal symptoms after menopause. -

Management of Women with Premature Ovarian Insufficiency
Management of women with premature ovarian insufficiency Guideline of the European Society of Human Reproduction and Embryology POI Guideline Development Group December 2015 1 Disclaimer The European Society of Human Reproduction and Embryology (hereinafter referred to as 'ESHRE') developed the current clinical practice guideline, to provide clinical recommendations to improve the quality of healthcare delivery within the European field of human reproduction and embryology. This guideline represents the views of ESHRE, which were achieved after careful consideration of the scientific evidence available at the time of preparation. In the absence of scientific evidence on certain aspects, a consensus between the relevant ESHRE stakeholders has been obtained. The aim of clinical practice guidelines is to aid healthcare professionals in everyday clinical decisions about appropriate and effective care of their patients. However, adherence to these clinical practice guidelines does not guarantee a successful or specific outcome, nor does it establish a standard of care. Clinical practice guidelines do not override the healthcare professional's clinical judgment in diagnosis and treatment of particular patients. Ultimately, healthcare professionals must make their own clinical decisions on a case-by-case basis, using their clinical judgment, knowledge, and expertise, and taking into account the condition, circumstances, and wishes of the individual patient, in consultation with that patient and/or the guardian or carer. ESHRE makes no warranty, express or implied, regarding the clinical practice guidelines and specifically excludes any warranties of merchantability and fitness for a particular use or purpose. ESHRE shall not be liable for direct, indirect, special, incidental, or consequential damages related to the use of the information contained herein. -

Rominder P.S. Suri, Phd, PE Robert F. Chimchirian & Sandhya Meduri Department of Civil & Environmental Engineering Villa
Emerging Contaminants and Water Supply – Speaker Abstracts Emerging Contaminants and Water Supply – Speaker Abstracts PRESENCE OF ESTROGEN HORMONES AND ANTIBIOTICS IN detection limits for all 12 estrogen compounds ranged from 0.1 ng L-1 to THE ENVIRONMENT 10 ng L-1 using a sample size of 1 L. Eight antibiotics and venlaflaxine Rominder P.S. Suri, PhD, PE were detected in the effluent with concentrations that ranged from 50 to Robert F. Chimchirian & Sandhya Meduri 2,930 ng L-1. Department of Civil & Environmental Engineering Twenty-one streams in Chester County, southeast Pennsylvania, were Villanova University, Villanova PA 19085 also sampled for aqueous concentrations of the above hormones and 610-519-7631 [email protected] antibiotics. Chester County represents a very diverse land use. In addition to concentrated urban developments, there are many In recent years, the detection of trace amount of pharmaceutically active agricultural farms (dairy, cattle, hog, poultry, and mushroom). The compounds (PACs) in the surface water has gained widespread watershed is comprised of about 280,000 acres that eventually drain into attention. The presence of the PACs in the natural water systems causes the Chesapeake Bay via the Susquehanna River. The results showed that ecological problems and possible adverse effects to humans. Two classes there was at least one estrogen detected in every stream sampled and six of PACs, estrogen hormones and antibiotics make their way into the estrogens were detected at three different locations. The most abundant surface waters through effluents from wastewater treatment plants and PAC was estrone, which was detected in all but one stream, with areas where animal manure and biosolids are land applied, amongst concentrations as high as 2.4 ng L-1. -

A Pharmaceutical Product for Hormone Replacement Therapy Comprising Tibolone Or a Derivative Thereof and Estradiol Or a Derivative Thereof
Europäisches Patentamt *EP001522306A1* (19) European Patent Office Office européen des brevets (11) EP 1 522 306 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.7: A61K 31/567, A61K 31/565, 13.04.2005 Bulletin 2005/15 A61P 15/12 (21) Application number: 03103726.0 (22) Date of filing: 08.10.2003 (84) Designated Contracting States: • Perez, Francisco AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 08970 Sant Joan Despi (Barcelona) (ES) HU IE IT LI LU MC NL PT RO SE SI SK TR • Banado M., Carlos Designated Extension States: 28033 Madrid (ES) AL LT LV MK (74) Representative: Markvardsen, Peter et al (71) Applicant: Liconsa, Liberacion Controlada de Markvardsen Patents, Sustancias Activas, S.A. Patent Department, 08028 Barcelona (ES) P.O. Box 114, Favrholmvaenget 40 (72) Inventors: 3400 Hilleroed (DK) • Palacios, Santiago 28001 Madrid (ES) (54) A pharmaceutical product for hormone replacement therapy comprising tibolone or a derivative thereof and estradiol or a derivative thereof (57) A pharmaceutical product comprising an effec- arate or sequential use in a method for hormone re- tive amount of tibolone or derivative thereof, an effective placement therapy or prevention of hypoestrogenism amount of estradiol or derivative thereof and a pharma- associated clinical symptoms in a human person, in par- ceutically acceptable carrier, wherein the product is pro- ticular wherein the human is a postmenopausal woman. vided as a combined preparation for simultaneous, sep- EP 1 522 306 A1 Printed by Jouve, 75001 PARIS (FR) 1 EP 1 522 306 A1 2 Description [0008] The review article of Journal of Steroid Bio- chemistry and Molecular Biology (2001), 76(1-5), FIELD OF THE INVENTION: 231-238 provides a review of some of these compara- tive studies. -

Use of Postmenopausal Hormone Therapy and Risk of Alzheimer’S BMJ: First Published As 10.1136/Bmj.L665 on 6 March 2019
RESEARCH Use of postmenopausal hormone therapy and risk of Alzheimer’s BMJ: first published as 10.1136/bmj.l665 on 6 March 2019. Downloaded from disease in Finland: nationwide case-control study Hanna Savolainen-Peltonen,1,2 Päivi Rahkola-Soisalo,1 Fabian Hoti,3 Pia Vattulainen,3 Mika Gissler,4,5,6 Olavi Ylikorkala,1 Tomi S Mikkola1,2 1University of Helsinki and ABSTRACT ratio 1.09, 95% confidence interval 1.05 to 1.14) and Helsinki University Hospital, OBJECTIVES those of oestrogen-progestogen (1.17, 1.13 to 1.21). Obstetrics and Gynecology, To compare the use of hormone therapy between The risk increases in users of oestrogen-progestogen Haartmaninkatu 2, PO Box 140, FIN-00029 HUS, 00029 Finnish postmenopausal women with and without a therapy were not related to different progestogens Helsinki, Finland diagnosis for Alzheimer’s disease. (noreth isterone acetate, medroxyprogesterone 2 Folkhälsan Research Center, DESIGN acetate, or other progestogens); but in women Biomedicum, Helsinki, Finland younger than 60 at hormone therapy initiation, 3 Nationwide case-control study. EPID Research Oy, Espoo, these risk increases were associated with hormone Finland SETTING therapy exposure over 10 years. Furthermore, the 4National Institute for Health Finnish national population and drug register, age at initiation of systemic hormone therapy was and Welfare, Helsinki, Finland between 1999 and 2013. 5Karolinska Institute, not a decisive determinant for the increase in risk Department of Neurobiology, PARTICIPANTS of Alzheimer’s disease. The exclusive use of vaginal Care Sciences and Society, All postmenopausal women (n=84 739) in Finland estradiol did not affect the risk of the disease (0.99, Division of Family Medicine, who, between 1999 and 2013, received a diagnosis of 0.96 to 1.01).