Leaf Rust / Brown Rust (Puccinia Triticina) Inoculation Protocol 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Additions to the Rust Fungi of South Africa
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by RERO DOC Digital Library Mycol Progress (2012) 11:483–497 DOI 10.1007/s11557-011-0764-z ORIGINAL ARTICLE Additions to the rust fungi of South Africa Reinhard Berndt & Alan R. Wood Received: 7 February 2011 /Revised: 15 April 2011 /Accepted: 19 April 2011 /Published online: 28 May 2011 # German Mycological Society and Springer 2011 Abstract This paper presents new species, combinations, Leucosidea sericea (Rosaceae), Uromyces cypericola whose national reports and host records for the South African rust urediniospores are described for the first time, Phakopsora fungi (Uredinales/Pucciniales). Endophyllum mpenjatiense stratosa in that spermogonia and Uredo-like aecia were on cf. Hibiscus sp. (Malvaceae), Phakopsora combretorum discovered, and for Sphaerophragmium dalbergiae in that (anamorph Uredo combreticola) on the new host Combretum characters of the urediniospores are re-evaluated. A lectotype apiculatum (Combretaceae) and Uredo sekhukhunensis on is selected for Aecidium garckeanum and spermogonia are Ziziphus mucronata (Rhamnaceae) are described as new reported for this rust for the first time. The rust fungi of species. Dietelia cardiospermi and E. metalasiae are Ehrharta (Poaceae) are discussed and critically evaluated in proposed as new combinations to replace Aecidium cardio- the light of spore morphology and host species. spermi on Cardiospermum halicacabum (Sapindaceae) and A. metalasiae on Metalasia spp. (Asteraceae), respectively. Keywords Combretum . Dietelia . Ehrharta . Four species are new records for South Africa: Crossopsora Endophyllum . Ziziphus antidesmae-dioicae on Antidesma venosum (Euphorbiaceae), Phakopsora ziziphi-vulgaris on Z. mucronata,andUromyces cypericola and Puccinia subcoronata, both on a new host, Introduction Cyperus albostriatus (Cyperaceae). -

Genome-Wide Association Study for Crown Rust (Puccinia Coronata F. Sp
ORIGINAL RESEARCH ARTICLE published: 05 March 2015 doi: 10.3389/fpls.2015.00103 Genome-wide association study for crown rust (Puccinia coronata f. sp. avenae) and powdery mildew (Blumeria graminis f. sp. avenae) resistance in an oat (Avena sativa) collection of commercial varieties and landraces Gracia Montilla-Bascón1†, Nicolas Rispail 1†, Javier Sánchez-Martín1, Diego Rubiales1, Luis A. J. Mur 2 , Tim Langdon 2 , Catherine J. Howarth 2 and Elena Prats1* 1 Institute for Sustainable Agriculture – Consejo Superior de Investigaciones Científicas, Córdoba, Spain 2 Institute of Biological, Environmental and Rural Sciences, University of Aberystwyth, Aberystwyth, UK Edited by: Diseases caused by crown rust (Puccinia coronata f. sp. avenae) and powdery mildew Jaime Prohens, Universitat Politècnica (Blumeria graminis f. sp. avenae) are among the most important constraints for the oat de València, Spain crop. Breeding for resistance is one of the most effective, economical, and environmentally Reviewed by: friendly means to control these diseases. The purpose of this work was to identify elite Soren K. Rasmussen, University of Copenhagen, Denmark alleles for rust and powdery mildew resistance in oat by association mapping to aid Fernando Martinez, University of selection of resistant plants. To this aim, 177 oat accessions including white and red oat Seville, Spain cultivars and landraces were evaluated for disease resistance and further genotyped with Jason Wallace, Cornell University, USA 31 simple sequence repeat and 15,000 Diversity ArraysTechnology (DArT) markers to reveal association with disease resistance traits. After data curation, 1712 polymorphic markers *Correspondence: Elena Prats, Institute for Sustainable were considered for association analysis. Principal component analysis and a Bayesian Agriculture – Consejo Superior de clustering approach were applied to infer population structure. -

Puccinia Sorghi), in Maize (Zea Mays
Emirates Journal of Food and Agriculture. 2020. 32(1): 11-18 doi: 10.9755/ejfa.2020.v32.i1.2053 http://www.ejfa.me/ RESEARCH ARTICLE Induced resistance to common rust (Puccinia sorghi), in maize (Zea mays) Carmen Alicia Zúñiga-Silvestre1, Carlos De-León-García-de-Alba1*, Victoria Ayala-Escobar1, Víctor A. González-Hernández2 1Instituto de Fitosanidad, Colegio de Postgraduados, Carretera México-Texcoco Km 36.5, Montecillo, Texcoco, Estado de México, C.P. 56230, México, 2Instituto de Fisiología Vegetal, Colegio de Postgraduados, Carretera México-Texcoco Km 36.5, Montecillo, Texcoco, Estado de México, C.P. 56230, México ABSTRACT The common rust of maize (Zea mays L.), caused by Puccinia sorghi Schw., develops pustules on the leaves of maize plants, reducing the leaf area and production of the photoassimilates necessary for grain filling. The host possesses genes coding for different proteins related to the defense mechanisms that prevent the establishment of the pathogen. However, there are susceptible plants that are unable of preventing pathogen attack. This condition depend on biotic and abiotic factors known as inducers of resistance which are able of activating the physico-chemical or morphological defense processes to counteract the invasion of the pathogen. The Ceres XR21 maize hybrid is susceptible to P. sorghi. In this work, maize hybrid was evaluated under a split-split- plot design established in two spring-autumn cycles in the years 2016 and 2017, in which five commercial products of biological and chemical origin reported as inducers of resistance, plus a fungicide were compared. The results showed that trifloxystrobin + tebuconazole (Consist Max®), sprayed on the foliage with 1.5X the commercially recommended dose, showed significant better response in most evaluated variables, because it controlled better the pathogen P. -

Rust Disease of Water Willow Intercepted in Import Plant Quarantine in Japan
RES.BULL.PL.PROT.JAPAN No. 41: 59~64(2005) Short Communication Rust Disease of Water Willow Intercepted in Import Plant Quarantine in Japan Yoichi MOTOKURA, Masayoshi NAGASE*, Akihiro OOI**, Koshi UEDA, and SHIGERU KIMURA Research Division, Yokohama Plant Protection Station 1-16-10, Shin-yamashita, Naka-ku, Yokohama 231- 0801, Japan. * Nagoya Airport Branch, Nagoya Plant Protection Station ** Nagoya Plant Protection Station Abstract: A rust disease on water willow(Justicia gendarussa Burm f.)was found at an import plant quar- antine inspection at Nagoya airport, in January, 2002. The causal rust fungus was identified with Puccinia thwaitesii Berk., based on it's morphology and the results of inoculation experiments. This is the first report on the interception of rust disease of water willow caused by P. thwaitesii at import plant quarantine inspection in Japan. Key words: rust, water willow, Justicia gendarussa, Puccinia thwaitesii Introduction Water willow(Justicia gendarussa Burm f..)is a perennial shrub native to the tropical and sub- tropical zones of the Asia, and it belongs to Acanthaceae(Editorial Committee of the Flora of Taiwan,1998). In the Southeast Asia, this plant is utilized as a raw material of Chinese medicine, or as a medicine for rheumatism(IWATSUKI et. al. ed., 1997). In our country, water willow is introduced and used as an ornamental plant, mainly for indoor. In January 2002, potted plants of water willow infected with a rust disease were found at an import plant quarantine inspection at Komaki(Nagoya international airport)in Japan. They were plants imported from Thailand for use as the ornamental foliage. -

Colours in Nature Colours
Nature's Wonderful Colours Magdalena KonečnáMagdalena Sedláčková • Jana • Štěpánka Sekaninová Nature is teeming with incredible colours. But have you ever wondered how the colours green, yellow, pink or blue might taste or smell? What could they sound like? Or what would they feel like if you touched them? Nature’s colours are so wonderful ColoursIN NATURE and diverse they inspired people to use the names of plants, animals and minerals when labelling all the nuances. Join us on Magdalena Konečná • Jana Sedláčková • Štěpánka Sekaninová a journey to discover the twelve most well-known colours and their shades. You will learn that the colours and elements you find in nature are often closely connected. Will you be able to find all the links in each chapter? Last but not least, if you are an aspiring artist, take our course at the end of the book and you’ll be able to paint as exquisitely as nature itself does! COLOURS IN NATURE COLOURS albatrosmedia.eu b4u publishing Prelude Who painted the trees green? Well, Nature can do this and other magic. Nature abounds in colours of all shades. Long, long ago people began to name colours for plants, animals and minerals they saw them in, so as better to tell them apart. But as time passed, ever more plants, animals and minerals were discovered that reminded us of colours already named. So we started to use the names for shades we already knew to name these new natural elements. What are these names? Join us as we look at beautiful colour shades one by one – from snow white, through canary yellow, ruby red, forget-me-not blue and moss green to the blackest black, dark as the night sky. -

Rust-Red Stringy Rot and Red Heart Rot Indian Paint Fungus and Bleeding Stereum in Firs
Rust-Red Stringy Rot and Red Heart Rot Indian paint fungus and bleeding Stereum in firs Pathogen—Rust-red stringy rot is caused by Echinodontium tinctorium. It is one of the few pathogens that has a common name—Indian paint fungus. Red heart rot is caused by Stereum (Haematostereum) sanguinolentum, also known as the bleeding Stereum. Hosts—Indian paint fungus attacks firs, hemlocks, and less commonly other species in western NorthAmerica, but it is primarily found on white fir in the Rocky Mountain Region. Bleeding Stereum occurs primarily on subal- pine fir and Engelmann spruce in this Region, though it can also attack other conifers. Signs and Symptoms—Indian paint fungus produces conks frequently at branch stubs, at wounds, and even inside hollow stems. The conk is hard and woody, hoof-shaped, and perennial (figs. 1-3). The upper surface is blackish and rough with crevices. The lower surface is grey-brown with hard, blunt, thick teeth. The inside is brilliant brick- or rust-red, but it fades over time after exposure. This tissue was ground into a powder and used as paint by some Native Americans, giving the fungus its name. The only other indicator of this disease is punk knots known as “rusty knots.” Although there is no swelling as with punk knots caused by Porodaedalea pini, the interior of the knot shows the rust-red color characteristic of the decay. Bleeding Stereum fruits frequently on logs and slash, but infrequently on live trees. Conks are small, thin, incon- spicuous, and leathery (fig. 4). Portions are appressed to the bark, often with a projecting cap. -

TCJP an Improved Method to Quantify <I>Puccinia Coronata</I> F
CORE Metadata, citation and similar papers at core.ac.uk Provided by UNL | Libraries University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Department of Agriculture: Agricultural Publications from USDA-ARS / UNL Faculty Research Service, Lincoln, Nebraska 2010 TCJP An improved method to quantify Puccinia coronata f. sp. avenae DNA in the host Avena sativa M. Acevedo USDA-ARS, [email protected] E. W. Jackson USDA-ARS A. Sturbaum USDA-ARS H. W. Ohm Purdue University J. M. Bonman USDA-ARS Follow this and additional works at: https://digitalcommons.unl.edu/usdaarsfacpub Part of the Agricultural Science Commons Acevedo, M.; Jackson, E. W.; Sturbaum, A.; Ohm, H. W.; and Bonman, J. M., "TCJP An improved method to quantify Puccinia coronata f. sp. avenae DNA in the host Avena sativa" (2010). Publications from USDA- ARS / UNL Faculty. 509. https://digitalcommons.unl.edu/usdaarsfacpub/509 This Article is brought to you for free and open access by the U.S. Department of Agriculture: Agricultural Research Service, Lincoln, Nebraska at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Publications from USDA-ARS / UNL Faculty by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Can. J. Plant Pathol. (2010), 32(2): 215–224 Genetics and resistance/Génétique et résistance AnTCJP improved method to quantify Puccinia coronata f. sp. avenae DNA in the host Avena sativa M.Crown rust of oat ACEVEDO1, E. W. JACKSON1, A. STURBAUM1, H. W. OHM2 AND J. M. BONMAN1 1USDA-ARS Small Grains and Potato Germplasm Research Unit, 1691 S. 2700 W., Aberdeen, ID 83210, USA 2Department of Agronomy, Purdue University, West Lafayette, IN 47907, USA (Accepted 1 March 2010) Abstract: Identification and genetic mapping of loci conferring resistance to polycyclic pathogens such as the rust fungi depends on accurate measurement of disease resistance. -

Color Chart.Pdf
® Finishing Products Division of RPM Wood Finishes Group Inc. Color Chart The Original Touch Up Company™ Made in the USA Color Chart ® Finishing Products Division of RPM Wood Finishes Group, Inc. Index Aerosols 1-5 Ultra® Classic Toner & Tone Finish Toner 1-3 Colored Lacquer Enamel 3-5 Shadow Toner 5 Touch-Up Markers/Pencils 5-15 Ultra® Mark Markers 5-9 3 in 1 Repair Stick 9 Pro-Mark® Markers 9-10 Quik-Tip™ Markers 10-11 Background Marker Touch-Up & Background Marker Glaze Hang-Up 11-13 Artisan Glaze Markers 13 Vinyl Marker Glaze Hang-Up 14 Brush Tip Graining Markers 14 Accent Pencils 15 Blend-Its 15 Fillers 15-29 Quick Fill® Burn-In Sticks 15-16 Edging/Low Heat Sticks 16 E-Z Flow™ Burn-In Sticks 16-17 PlaneStick® Burn-In Sticks 17-18 Fil-Stik® Putty Sticks 18-25 Hard Fill & Hard Fill Plus 25-27 PermaFill™ 27 Epoxy Putty Sticks 27-28 Patchal® Puttys 28-29 Knot Filler 29 Fil-O-Wood™ Wood Putty Tubes 29 Color Replacement 30-31 Blendal® Sticks 30 Sand Thru Sticks 30-31 Blendal® Powder Stains 31 Bronzing Powders 31 Dye Stains 32 Ultra® Penetrating & Architectural Ultra® Penetrating Stain 32 Dye Concentrate 32 Pigmented Stains 32-34 Wiping Wood™, Architectural Wiping Stain & Wiping Wood™ Stain Aerosols 32-33 Designer Series Stain, Designer Series Radiant Stain 33-34 Glazes 34 Finisher’s Glaze™ Glazing Stain & Aerosols 34 Break-A-Way™ Glaze & Aerosols 34 Leather Repair 35-37 E-Z Flow™ Leather Markers 35 Leather/Vinyl Markers 35 Leather/Vinyl Fil Sticks 35-36 Leather Repair Basecoat Aerosols 36 Leather Repair Toner Aerosols 36 Leather Repair Color Adjuster Aerosols 37 Touch Up Pigment 37 Leather Refinishing 37 Base Coat 37 NOTE: COLORS ARE APPROXIMATE REPRESENTATIONS OF ACTUAL COLORS USING MODERN PROCESS TECHNIQUES. -

Integrated Management of Southern Corn Rust and Northern Corn
INTEGRATED MANAGEMENT OF SOUTHERN CORN RUST AND NORTHERN CORN LEAF BLIGHT USING HYBRIDS AND FUNGICIDES by SUZETTE MAGDALENE SEÑEREZ ARCIBAL (Under the Direction of Robert C. Kemerait, Jr.) ABSTRACT Southern corn rust (SCR) caused by Puccinia polysora and northern corn leaf blight (NCLB) caused by Exserohilum turcicum are important foliar diseases of corn in the southern United States. Field experiments were conducted to determine the effect of hybrid, fungicide and timing of fungicide application on NCLB and SCR epidemics and corn yield. The Rpp9-virulent and Rpp9-avirulent races of P. polysora were characterized in the field. Onset of SCR in Pioneer 33M52 was delayed in early-planted trials but not in later-planted trials. Area under the disease progress curves (AUDPC) for SCR were lower and yields were higher in Pioneer 33M52 than in Pioneer 33M57 when this disease was severe. Fungicides were usually most effective when applied near disease onset. When both diseases were severe, multiple fungicide applications improved disease management and yield. In vitro sensitivity assays indicated a range of EC50 values from 0.008 to 0.155 μg/ml. These results can be used to further develop management guidelines for SCR and NCLB. INDEX WORDS: Southern corn rust, Puccinia polysora, Rpp9-virulent race, northern corn leaf blight, Exserohilum turcicum, pyraclostrobin, metconazole, fluxapyroxad, fungicide timing, area under the disease progress curve, severity, incidence, necrosis, yield, fungicide sensitivity INTEGRATED MANAGEMENT OF SOUTHERN CORN -

Rust Diseases of Brambles
University of Kentucky College of Agriculture, Food & Environment Extension Plant Pathology College of Agriculture, Food and Environment Cooperative Extension Service Plant Pathology Fact Sheet PPFS-FR-S-06 Rust Diseases of Brambles Nicole Gauthier Jessica Sayre Plant Pathology Horticulture Extension Specialist Extension Agent Importance Cane & Leaf Rust The three most important rust diseases occurring Symptoms & Signs on brambles in Kentucky are cane and leaf rust, late The first evidence of cane and leaf rust is the presence rust, and orange rust. The most destructive of these of elongated, bright yellow pustules appearing on diseases is orange rust, which is ultimately lethal to infected floricanes (year-old canes that will produce plants. Once infected, entire plants must be removed fruit) in spring (Figure 1). Pustules rupture through and destroyed. In contrast, cane and leaf rust, along the bark and result in brittle canes that break easily. with late rust, are not lethal to plants and can be Small yellow pustules may also appear on undersides managed using cultural practices and fungicides. of leaves (Figure 2) and less frequently on fruit Distinguishing between these rust diseases is critical (Figure 3). Fungal signs (pustules of powdery yellow for proper management. rust spores) may be evident in mid-April and extend through summer. Premature defoliation, which results in stress and loss of plant vigor, can occur if 1a leaf infections are severe. Hosts Blackberry is susceptible; raspberry infections are rare. 1a Figure 1. (A) Cane and leaf rust pustules erupt through the bark of floricanes in spring. (B) Close-up of cane and leaf rust pustule containing abundant powdery yellow spores. -
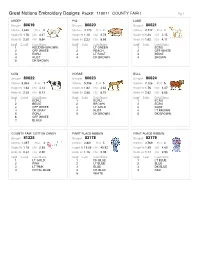
J9 Packline Report V8
Great Notions Embroidery Designs Pack#: 110011 COUNTY FAIR I Pg 1 SHEEP PIG LAMB Design# 80619Design# 80620Design# 80621 Stitches 4,622 #Col 5 Stitches 4,170 #Col 4 Stitches 2,747 #Col 4 Height IN 1.76 CM 4.47 Height IN 1.10 CM 2.79 Height IN 1.24 CM 3.15 Width IN 2.30 CM 5.66 Width IN 2.23 CM 5.66 Width IN 1.62 CM 4.11 Seq# Color# Color Name Seq# Color Color Name Seq# Color Color Name 1 REDDISH BROWN 1 LT GREEN 1 ECRU 2 OFF WHITE 2 PEACH 2 OFF WHITE 3 ECRU 3 LT RUST 3 BEIGE 4 RUST 4 DK BROWN 4 BROWN 5 DK BROWN COW HORSE BULL Design# 80622Design# 80623Design# 80624 Stitches 5,834 #Col 7 Stitches 5,756 #Col 5 Stitches 7,126 #Col 5 Height IN 1.63 CM 4.14 Height IN 1.82 CM 4.62 Height IN 1.76 CM 4.47 Width IN 2.35 CM 6.73 Width IN 2.65 CM 6.73 Width IN 2.62 CM 6.65 Seq# Color# Color Name Seq# Color Color Name Seq# Color Color Name 1 ECRU 1 ECRU 1 ECRU 2 BEIGE 2 BROWN 2 ECRU 3 OFF WHITE 3 LT GOLD 3 RUST 4 DK GRAY 4 RUST 4 LT BROWN 5 ECRU 5 DK BROWN 5 DK BROWN 6 OFF WHITE 7 BLACK 'COUNTY FAIR' COTTON CANDY FIRST PLACE RIBBON FIRST PLACE RIBBON Design# 81325Design# 83178Design# 83179 Stitches 1,397 #Col 4 Stitches 3,801 #Col 5 Stitches 3,769 #Col 4 Height IN 1.16 CM 2.95 Height IN 18.08 CM 45.92 Height IN 1.89 CM 4.80 Width IN 2.34 CM 2.95 Width IN 1.16 CM 2.95 Width IN 1.14 CM 2.90 Seq# Color# Color Name Seq# Color Color Name Seq# Color Color Name 1 LT GOLD 1 DK BLUE 1 LT BLUE 2 PINK 2 LT BLUE 2 BLUE 3 LT PINK 3 BLUE 3 DK BLUE 4 ROYAL BLUE 4 DK BLUE 4 RED 5 WHITE Great Notions Embroidery Designs Pack#: 110011 COUNTY FAIR I Pg 2 FIRST PLACE -

Stem Rust on Wheat and Barley
Identification and Management of Stem Rust on Wheat and Barley Stem rust, leaf rust, and stripe rust comprise a production. The first of these races, known as ‘Ug99’, complex of diseases that reduces wheat and barley was originally detected in Uganda, Kenya, and grain production. These rust diseases occur in nearly Ethiopia. Since this initial detection, additional races all areas of the United States and Canada. The of the fungus have been reported and are further importance of any member of the complex at a given complicating efforts to contain the problem. location is determined by specific interactions with If these new races spread to North America, current wheat varieties, they may threaten wheat crop growth stage, and and barley production. weather conditions. Why does the genetic In preparation for the Stem rust has been resistance appear to fail? possible introduction of present in North America Currently genetic resistance is an effective these new races of stem for hundreds of years. means of managing the rust diseases of rust, a number of critical A series of particularly wheat and barley. Because the populations questions arise regarding severe outbreaks occurred the most effective ways during the early 1930s and of the fungi that cause rust diseases can to identify, monitor, and 1950s. These outbreaks change and adapt to the resistance genes of manage the disease. This caused serious yield loss in current varieties, the durability of this genetic publication answers these many parts of the United resistance has been problematic. These critical questions with the States and Canada with changes occur when naturally occurring best available information the greatest losses in the genetic changes allow members of the about the emerging threat.