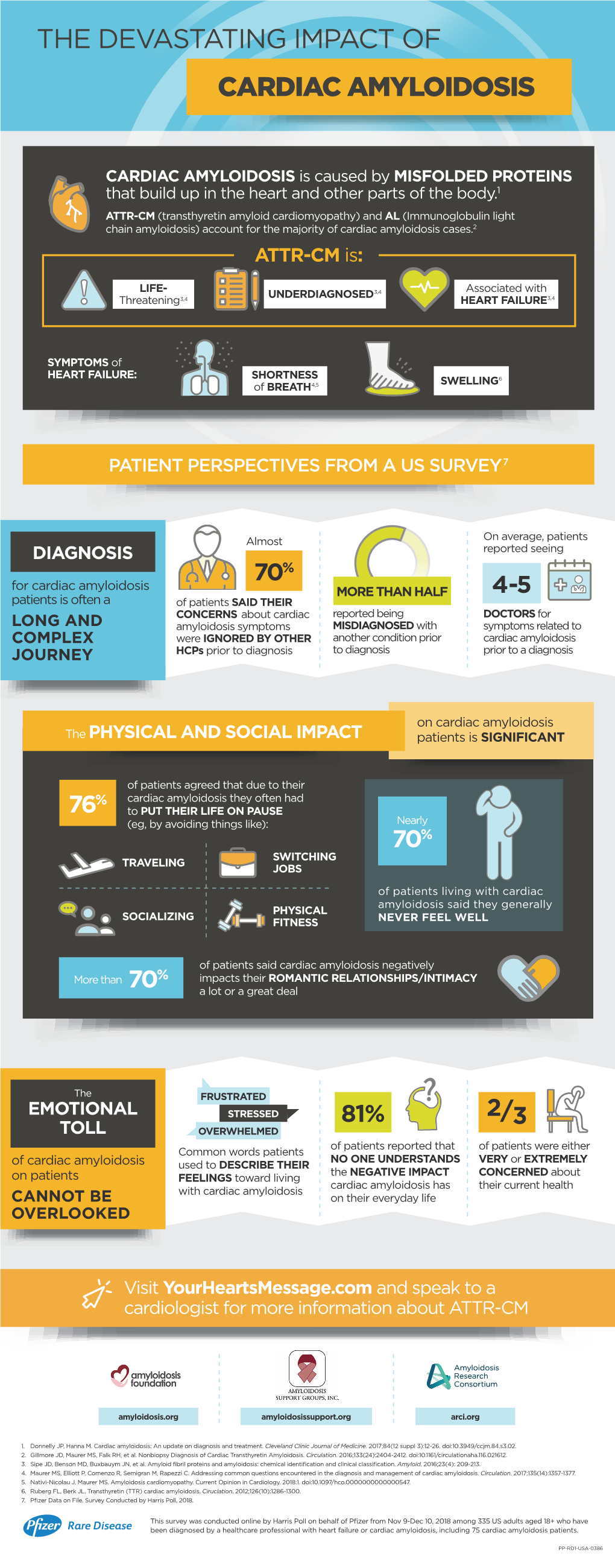
The Devastating Impact of Cardiac Amyloidosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cardiac Amyloidosis
Cardiac Amyloid: Contemporary Approach to Diagnosis and Advances in Treatment Cardiac Nursing Symposium October 17, 2019 Dana Miller AGPCNP-BC, CHFN The University of Kansas Medical Center Heart Failure Nurse Practitioner Oc Octob Learning objectives • Describe the different types of cardiac amyloidosis (Disease) • Recognize clinical manifestations of cardiac amyloidosis • Implement strategies for diagnosis of cardiac amyloidosis (Diagnosis) • Utilize recent clinical evidence for decisions about treatment of cardiac amyloidosis (Drugs and devices) What is the disease amyloidosis? • First described by Rudolf Virchow in 1858 describing the reaction of tissue deposits with iodine and sulfuric acid. • A disorder of misfolded proteins • Proteins circulate in the bloodstream and perform many functions in the body • Should be dissolved, in other words, liquid • In amyloid they become solid and deposit in organs and tissues in the body and cause problems Red flags for Cardiac Amyloidosis • Echocardiography • Low voltage on ECG and thickening of the septum/posterior wall>1.2 cm (unexplained increase in thickness) • Thickening of the RV free wall, valves Intolerance to bet blockers or ACEI Low normal BP inpatients with a previous history of HTN Donnelly and Hanna, 2017 JACC 2014 Red flags for AL • HFpEF + Nephrotic syndrome • Macroglossia and/or periorbital purpura • Orthostatic hypotension • Peripheral neuropathy • MGUS • Donnelly and Hanna, 2017 Red flags for ATTR • White male age>60 with HFpEF + history of carpal tunnel syndrome and or/spinal -

Recent Advances in the Diagnosis and Management of Amyloid Cardiomyopathy
Faculty Opinions Faculty Reviews 2021 10:(31) Recent advances in the diagnosis and management of amyloid cardiomyopathy Petra Nijst 1,2 W.H. Wilson Tang 3* 1 Department of Cardiology, Ziekenhuis Oost-Limburg, Genk, Belgium 2 Biomedical Research Institute, Faculty of Medicine and Life Sciences, Hasselt University, Diepenbeek, Belgium 3 Department of Cardiovascular Medicine, Heart and Vascular Institute, Cleveland Clinic, Cleveland, OH, USA Abstract Amyloidosis is a disorder characterized by misfolded precursor proteins that form depositions of fibrillar aggregates with an abnormal cross-beta-sheet conformation, known as amyloid, in the extracellular space of several tissues. Although there are more than 30 known amyloidogenic proteins, both hereditary and non-hereditary, cardiac amyloidosis (CA) typically arises from either misfolded transthyretin (ATTR amyloidosis) or immunoglobulin light-chain aggregation (AL amyloidosis). Its prevalence is more common than previously thought, especially among patients with heart failure and preserved ejection fraction (HFpEF) and aortic stenosis. If there is a clinical suspicion of CA, focused echocardiography, laboratory screening for the presence of a monoclonal protein (serum and urinary electrophoresis with immunofixation and serum free light-chain ratio), and cardiac scintigraphy with 99mtechnetium-labeled bone-tracers are sensitive and specific initial diagnostic tests. In some cases, more advanced/invasive techniques are necessary and, in the last several years, treatment options for both AL CA and ATTR CA have rapidly expanded. It is important to note that the aims of therapy are different. Systemic AL amyloidosis requires treatment targeted against the abnormal plasma cell clone, whereas therapy for ATTR CA must be targeted to the production and stabilization of the TTR molecule. -

Aortic Stenosis and Transthyretin Cardiac Amyloidosis: the Chicken Or the Egg?
European Heart Journal Advance Access published February 22, 2016 European Heart Journal EHJ BRIEF COMMUNICATION doi:10.1093/eurheartj/ehw033 Valvular heart disease Aortic stenosis and transthyretin cardiac amyloidosis: the chicken or the egg? Arnault Galat1,2,3,4,5, Aziz Guellich1,2,3,4,5, Diane Bodez1,2,3,4,5, Michel Slama6, Marina Dijos7, David Messika Zeitoun8, Olivier Milleron8, David Attias9, Jean-Luc Dubois-Rande´ 1,2,3,4,5, Dania Mohty10, Etienne Audureau1,2,4,5,11,12, Emmanuel Teiger1,2,3,4,5, Jean Rosso1,2,13, Jean-Luc Monin1,2,3,4,5, and Thibaud Damy1,2,3,4,5* 1UPEC, Cre´teil F-94000, France; 2MondorAmyloidosis Network, Cre´teil F-94000, France; 3Department of Cardiology, AP-HP, Henri-Mondor Teaching Hospital, Cre´teil F-94000, Downloaded from France; 4INSERM U955, Cre´teil F-94000, France; 5DHU A-TVB, Cre´teil F-94000, France; 6Department of Cardiology, AP-HP, Antoine Be´cle`re Teaching Hospital, Clamart F-92140, France; 7Department of Cardiology, Bordeaux Teaching Hospital, Pessac F-33604, France; 8Department of Cardiology, AP-HP, Bichat Teaching Hospital, Paris F-75018, France; 9Department of Cardiology, Centre Cardiologique du Nord, Saint-Denis F-93200, France; 10Department of Cardiology, Dupuytren Teaching Hospital, Limoges F-87042, France; 11Department of Public Health, Henri-Mondor Teaching Hospital, Cre´teil F-94000, France; 12CEpiA (Clinical Epidemiology and Ageing), EA4393, Universite´ Paris Est (UPE), UPEC, F-94000, Cre´teil, France; and 13Department of Nuclear Medicine, AP-HP, Henri-Mondor Teaching Hospital, Cre´teil F-94000, France Received 8 July 2015; revised 18 November 2015; accepted 21 January 2016 http://eurheartj.oxfordjournals.org/ Background Aortic stenosis (AS) and transthyretin cardiac amyloidosis (TTR-CA) are both frequent in elderly. -

Transthyretin Amyloid Cardiomyopathy (ATTR-CM)—
BIOEQUIVALENCE / VYNDALINK / REFS When you’ve decided VYNDAMAX is appropriate for your patient, VyndaLink may be able to help ATTR-CM is a progressive, fatal Enroll your patients in VyndaLink for support or send prescriptions directly to an in-network Specialty Pharmacy disease—recognize the signs The VyndaLink team can: and symptoms to diagnose and treat 2,3 Conduct a benefits verification to Determine payer requirements and Identify Specialty Pharmacy options based your appropriate patients determine your patient’s coverage provide information about the prior on your patient’s insurance coverage. Transthyretin amyloid for VYNDAMAX, including out-of- authorization process and appeals VYNDAMAX is available through multiple pocket costs. process as needed.* Specialty Pharmacies in our defined distribution network. VYNDAMAX—once-daily, oral medication for patients with wild-type or hereditary ATTR-CM *Please note where a PA is required, the physician must submit required information directly to the patient’s insurer. cardiomyopathy (ATTR-CM)— Get started at www.VyndaLink.com a disease that may be present in Download the enrollment form. Completed form can be sent online at www.VyndaLinkPortal.com or Proven to reduce mortality and 1 AM PM patients with heart failure faxed to 1-888-878-8474. Call 1-888-222-8475 (Monday-Friday, 8 -8 ET) with any questions. CV-related hospitalization Consider prescribing oral VYNDAMAX for References: 1. Nativi-Nicolau J, Maurer MS. Amyloidosis cardiomyopathy: update in the diagnosis and treatment of the most common types. Curr Opin Cardiol. 2018;33(5):571-579. 2. Sipe JD, Benson MD, Buxbaum JN, et al. Amyloid bril proteins and amyloidosis: chemical identication and clinical classication International Society of Amyloidosis 2016 Nomenclature Guidelines. -

A Guide to Transthyretin Amyloidosis
A Guide to Transthyretin Amyloidosis Authored by Teresa Coelho, Bo-Goran Ericzon, Rodney Falk, Donna Grogan, Shu-ichi Ikeda, Mathew Maurer, Violaine Plante-Bordeneuve, Ole Suhr, Pedro Trigo 2016 Edition Edited by Merrill Benson, Mathew Maurer What is amyloidosis? Amyloidosis is a systemic disorder characterized by extra cellular deposition of a protein-derived material, known as amyloid, in multiple organs. Amyloidosis occurs when native or mutant poly- peptides misfold and aggregate as fibrils. The amyloid deposits cause local damage to the cells around which they are deposited leading to a variety of clinical symptoms. There are at least 23 different proteins associated with the amyloidoses. The most well-known type of amyloidosis is associated with a hematological disorder, in which amyloid fibrils are derived from monoclonal immunoglobulin light-chains (AL amyloidosis). This is associated with a clonal plasma cell disorder, closely related to and not uncommonly co-existing with multiple myeloma. Chronic inflammatory conditions such as rheumatoid arthritis or chronic infections such as bronchiectasis are associated with chronically elevated levels of the inflammatory protein, serum amyloid A, which may misfold and cause AA amyloidosis. The hereditary forms of amyloidosis are autosomal dominant diseases characterized by deposition of variant proteins, in dis- tinctive tissues. The most common hereditary form is transthyretin amyloidosis (ATTR) caused by the misfolding of protein monomers derived from the tetrameric protein transthyretin (TTR). Mutations in the gene for TTR frequently re- sult in instability of TTR and subsequent fibril formation. Closely related is wild-type TTR in which the native TTR protein, particu- larly in the elderly, can destabilize and re-aggregate causing non- familial cases of TTR amyloidosis. -

Angina with a Normal Coronary Angiogram Caused by Amyloidosis D C Whitaker, M F Tungekar, J E Dussek
1of2 CASE REPORT Heart: first published as 10.1136/hrt.2004.038984 on 13 August 2004. Downloaded from Angina with a normal coronary angiogram caused by amyloidosis D C Whitaker, M F Tungekar, J E Dussek ............................................................................................................................... Heart 2004;90:e54 (http://www.heartjnl.com/cgi/content/full/90/9/e54). doi: 10.1136/hrt.2004.038984 ganglion block) was performed with a good but temporary A case of severe intractable angina pectoris with normal result and on this basis the patient proceeded to have a video angiography is presented. Following video assisted thoracic assisted thoracoscopic (VATS) left sympathectomy. The left sympathectomy the patient died of heart failure. sympathetic chain was divided with diathermy over the Microvascular cardiac amyloidosis was diagnosed at the necks of the second, third, and fourth ribs. Three hours after postmortem examination. This report alerts clinicians to this the procedure the patient suddenly developed respiratory possible diagnosis when treating patients with severe angina failure (PO2 5.6 kPa and PCO2 3.1 kPa) with signs of when no cause is found and discusses the poor prognosis in pulmonary oedema. He was intubated, ventilated, and given such cases. an infusion of noradrenaline (norepinephrine) to maintain support. He was transferred to the intensive care unit that evening. The ECG remained normal. Troponin T was mildly increased (0.18 ng/ml, normal , 0.05 ng/ml). Echocar- arly amyloidosis without myocardial involvement can diogram showed mild global left ventricular dysfunction. produce severe anginal symptoms by obstructing the Ventilation was continued for eight days in the intensive care intramural (rather than the epicardial) coronary arteries. -

Cardiac Amyloidosis
Cardiac Amyloidosis Ronald Witteles, MD Stanford University & Brendan M. Weiss, MD University of Pennsylvania Amyloidosis: What is it? • Amylum – Starch (Latin) • Generic term for many diseases: • Protein misfolds into β-sheets • Forms into 8-10 nm fibrils • Extracellular deposition into amyloid deposits Types of Amyloid – Incomplete List • Systemic: • Light chains (AL) – “Primary ” • Transthyretin (ATTR) – “Senile ” or “Familial ” or “FAC” or “FAP” • Serum amyloid A (AA) – “Secondary ” • Localized – Not to be memorized! • Beta-2 microglobulin (A-β2) – Dialysis (osteoarticular structures) • Apolipoprotein A-1 (AApoA-I) – Age-related (aortic intima, cardiac, neuropathic) • Apolipoprotein A-2 (AApoA-2) – Hereditary (kidney) • Calcitonin (ACal) – Complication of thyroid medullary CA • Islet amyloid polypeptide (AIAPP) – Age-related (seen in DM) • Atrial natriuretic peptide (AANF) – Age-related (atrial amyloidosis) • Prolactin (APro) – Age-related, pituitary tumors • Insulin (AIns) – Insulin-pump use (local effects) • Amyloid precursor protein (ABeta) – Age-related/hereditary (Alzheimers) • Prion protein (APrPsc) – Hereditary/sporadic (spongiform encephalopathies) • Cystatin-C (ACys) – Hereditary (cerebral hemorrhage) • Fibrinogen alpha chain (AFib) – Hereditary (kidney) • Lysozome (ALys) – Hereditary (Diffuse, especially kidney, spares heart) • Medin/Lactadherin – Age-related (medial aortic amyloidosis) • Gelsolin (AGel) – Hereditary (neuropathic, corneal) • Keratin – Cutaneous AL: A Brief Dive into Hematology… Plasma cells: Make antibodies -

Microscopic Amyloid Deposits in the Heart Valves: a Common Local Complication of Chronic Damage and Scarring
J Clin Pathol: first published as 10.1136/jcp.33.3.262 on 1 March 1980. Downloaded from J Clin Pathol 1980; 33: 262-268 Microscopic amyloid deposits in the heart valves: a common local complication of chronic damage and scarring Y GOFFIN From the Department ofPathology, University Hospital Brugmann, Brussels, and the Universite Libre de Bruxelles, Brussels, Belgium SUMMARY The presence of amyloidosis was detected in 33 out of 213 (15-5 %) mitral and aortic valves that had been surgically removed for chronic valvular disease. No correlation could be found with age or type ofvalvular disease, neither was there any clinical evidence ofan associated generalised amyloidosis. Histologically, the amyloid deposits were microscopic and restricted to areas of dense scar tissue. No comparable alterations were found in 147 unaltered valves which served as controls. The term 'dystrophic' is proposed to describe this particular form of valvular amyloidosis. The deposition of amyloid in the heart valves is a The purpose of the present paper is to report well-known occurrence in generalised primary another type of valvular amyloidosis which appears amyloidosis and in senile cardiac amyloidosis. A in relation to local scarring. It was discovered copyright. survey of the literature concerning cardiac localisa- accidentally by histological examination of chronic- tions of amyloidosis shows that valvular involvement ally diseased heart valves which had been surgically is a relatively infrequent finding (Silwer and Lind- removed for valvular stenosis and/or incompetence. blom, 1926; Lubarsch, 1929; Koller, 1932; Israel, Subsequently we extended our investigation in 1933; Kerwin, 1936; Koletsky and Stecher, 1939; order to include adequate controls which were Dillon and Evans, 1942; Lindsay and Knorp, 1945; taken from necropsy material. -

Asnc Cardiac Amyloidosis Practice Points
ASNC CARDIAC AMYLOIDOSIS PRACTICE POINTS 99mTechnetium- Pyrophosphate Imaging for Transthyretin Cardiac Amyloidosis CARDIAC AMYLOIDOSIS ASNC PRACTICE POINTS 99mTechnetium-Pyrophosphate Imaging for Transthyretin Cardiac Amyloidosis OVERVIEW The purpose of this document is to identify the critical components involved in performing 99mTechnetium-pyrophosphate (99mTc-PYP) imaging for the evaluation of cardiac transthyretin amyloidosis (ATTR). BACKGROUND • The majority of individuals with cardiac amyloidosis have myocardial amyloid deposits formed from misfolded light chain (AL) or transthyretin (TTR) proteins. Diagnosis of amyloidosis and differentiation between the types is important for prognosis, therapy, and genetic counseling. • Cardiac ATTR amyloidosis, the focus of this practice points document, is an under diagnosed cause of heart failure. • Amyloid derived from wild-type TTR results in a restrictive cardiomyopathy, most commonly presenting in men in their early 70’s onwards, but occasionally seen as young as age 60. Although almost 1 in 4 males > 80 years have some TTR-derived amyloid deposits at autopsy, the clinical significance of a mild degree of deposition is unknown--generally clinical manifestations of heart failure occur once enough amyloid has been deposited to cause LV wall thickening (1). • Approximately 3 – 4% among US African Americans have a common inherited mutation of the TTR gene (Val122Ile), which produces a restrictive cardiomyopathy in a minority, but may contribute to heart failure in a higher proportion (1). • Cardiac amyloidosis should be suspected in individuals with heart failure and thickened ventricles with grade 2 or greater diastolic dysfunction on echocardiography or typical findings on cardiac magnetic resonance imaging (CMR; diffuse late gadolinium enhancement, ECV expansion or characteristic T-1 relaxation times); diagnosis is confirmed by endomyocardial biopsy and typing of amyloid fibrils as needed. -

Intraoperative Death Due to Nodular Amyloidosis Cardiomyopathy Associated with Fat Pulmonary Embolism
Rom J Leg Med [21] 15-18 [2013] DOI: 10.4323/rjlm.2013.15 © 2013 Romanian Society of Legal Medicine Intraoperative death due to nodular amyloidosis cardiomyopathy associated with fat pulmonary embolism Mihai Ceauşu1, Lăcrămioara Luca2, Sorin Hostiuc3,*, Dana Sîrbu4, Adrian Sîrbu4, Ruxandra Negoi5 _________________________________________________________________________________________ Abstract: Cardiac amyloidosis is caused by extracellular deposits containing low molecular weight protein subunits arranged in a beta sheet configuration. By gross examination amyloid deposits are identifiable as localized tan, waxy appearing lesions affecting almost always the atria (usually atrial endocardium), valve leaflets, ventricular, but also in the coronary lumen, sometimes leading to severe coronary stenosis. Fat pulmonary embolism is a known complication of femoral fractures, being more frequent in untreated cases compared to those suffering surgical interventions. We present a case in which a female patient with cardiac amyloidosis died on the operating table, the direct cause of death being fat pulmonary emboli, and discuss the involvement of the associated cardiac amyloidosis in thanatogenesys. Key Words: cardiac amyloidosis, fat embolism, intraoperative death, femoral fracture. ardiac amyloidosis (amyloid associated with fibrous replacement lesions), but also the cardiomyopathy, AC) is caused by coronary lumen, sometimes leading to severe coronary C extracellular deposits containing low molecular weight stenosis (>75%) [5]. protein subunits arranged in a beta sheet configuration, Cardiac amyloidosis is almost always associated leading to restrictive cardiomyopathy [1] and electrical with amyloid deposits located in other organs [5]. The conduction disturbances [2, 3]. extent of amyloid deposition can be graded from 1 to 4 AC may mimic constrictive pericarditis, coronary (less than 10%, 10 to 25%, 26 to 50%, and more than artery disease, valve heart disease, and idiopathic 50% involvement of the myocardium, respectively) hypertrophic or congestive cardiomyopathy [4]. -

Associations of Electrocardiographic Parameters with Left Ventricular
www.nature.com/scientificreports OPEN Associations of Electrocardiographic Parameters with Left Ventricular Longitudinal Received: 3 January 2019 Accepted: 7 May 2019 Strain and Prognosis in Cardiac Published: xx xx xxxx Light Chain Amyloidosis Darae Kim1, Ga Yeon Lee1, Jin-Oh Choi1, Kihyun Kim2, Seok Jin Kim2 & Eun-Seok Jeon1 A 12-lead ECG is a simple and less costly measure to assess cardiac amyloidosis and may refect the infltrative nature of cardiac amyloidosis and have prognostic value for predicting overall survival in patients with cardiac AL amyloidosis. Therefore, we investigated the associations of surface ECG parameters with left ventricular (LV) global longitudinal strain (GLS) and prognosis in patients with cardiac AL amyloidosis. We performed a multi-center, retrospective analysis of 102 biopsy-proven cardiac AL amyloidosis patients. Baseline studies included 12-lead surface ECG and echocardiography, with two-dimensional strain analysis performed within one month of diagnosis. From the Kaplan- Meier survival analysis, patients with prolonged QTc (≥483 msec) had signifcantly poorer survival. ECG scores were assigned according to presence of prolonged QTc (≥483 msec) and abnormal QRS axis, and the study participants were divided into three groups according to ECG score. Mean absolute value of LV GLS and regional LV longitudinal strain (LS) difered signifcantly among the three groups and decreased in a stepwise manner as ECG score increased. Log NT-proBNP increased in a stepwise manner as ECG score increased. Prolonged QTc (≥483 msec) and abnormal QRS axis showed signifcant incremental values in addition to the revised Mayo stage. The presence of prolonged QTc (≥483 msec) and abnormal QRS axis showed signifcant incremental values for overall mortality rates. -

Clinical Relevance of Pulmonary Amyloidosis: an Analysis of 76 Autopsy-Derived Cases
AGORA | RESEARCH LETTER Clinical relevance of pulmonary amyloidosis: an analysis of 76 autopsy-derived cases To the Editor: Amyloidosis refers to a group of diseases characterised by the extracellular tissue deposition of insoluble misfolded proteins that disrupt the function of the involved organs [1]. The principal types of systemic amyloidosis include immunoglobulin light-chain amyloidosis (AL) due to an underlying monoclonal B-lymphocyte/plasma cell disorder, serum amyloid A amyloidosis (AA) due to chronic inflammatory diseases, and transthyretin amyloidosis (ATTR) from variations (mutations) in the transthyretin gene (mut-ATTR) or acquired form, i.e. wild-type ATTR (wt-ATTR) [1]. Deaths related to amyloidosis are usually attributed to cardiac involvement with AL. The prevalence of pulmonary deposition in AL patients has ranged from 36% to 90% based on histopathologic studies, but clinical correlation has been sparse [2, 3]. To shed additional insights on this issue, we analysed autopsy cases of amyloidosis to ascertain the clinical relevance of pulmonary involvement, including its role in the death of these patients. We used a computer-assisted search of medical records to identify all patients diagnosed by autopsy to have pulmonary amyloidosis at our institution between January 1, 1997 and September 30, 2014. We reviewed relevant medical records, radiologic studies, and autopsy slides. Congo red and/or sulfated Alcian blue staining was used to identify amyloid deposition. The type of amyloid was determined by nanoflow liquid chromatography–tandem mass spectrometry as previously described [4]. The demographics of the 76 patients with autopsy-proven pulmonary amyloidosis are summarised in table 1. AL accounted for 76% of cases; nearly all were diagnosed to have amyloidosis antemortem.