Protocol Apatinib for Advanced Osteosarcoma After
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transarterial Chemoembolization (TACE) Combined with Apatinib
283 Original Article Page 1 of 14 Transarterial chemoembolization (TACE) combined with apatinib versus TACE combined with sorafenib in advanced hepatocellular carcinoma patients: a multicenter retrospective study Zhiyu Qiu1,2#^, Lujun Shen1,3#, Yiquan Jiang1,3,4#, Jiliang Qiu1,2#, Zining Xu5, Mengting Shi6, Zhentao Yu6, Yanping Ma7, Wei He1,2, Yun Zheng1,2, Binkui Li1,2, Guoying Wang4, Yunfei Yuan1,2^ 1State Key Laboratory of Oncology in South China and Collaborative Innovation Center of Cancer Medicine, Sun Yat-sen University, Guangzhou, China; 2Department of Liver Surgery, Sun Yat-sen University Cancer Center, Guangzhou, China; 3Department of Minimally Invasive Interventional Therapy, Sun Yat-sen University Cancer Center, Guangzhou, China; 4Department of Hepatic Surgery and Liver Transplantation Center, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China; 5Department of Minimally Invasive Interventional Radiology, the Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, China; 6Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, China; 7Department of Radiology, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China Contributions: (I) Conception and design: Y Yuan, G Wang, Z Qiu; (II) Administrative support: Y Yuan, G Wang, L Shen, Y Jiang, J Qiu; (III) Provision of study materials or patients: Y Yuan, G Wang, B Li, Y Zheng, J Qiu, W He, Z Xu; (IV) Collection and assembly of data: Z Qiu, Z Xu, M Shi, Z Yu, Y Ma; (V) Data analysis and interpretation: Z Qiu, J Qiu, W He, Y Zheng, B Li; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. #These authors contributed equally to this work. -

Apatinib Plus Ifosfamide and Etoposide for Relapsed Or Refractory Osteosarcoma: a Retrospective Study in Two Centres
ONCOLOGY LETTERS 22: 552, 2021 Apatinib plus ifosfamide and etoposide for relapsed or refractory osteosarcoma: A retrospective study in two centres LU XIE1, JIE XU1, XIN SUN1, XIAOWEI LI1, KUISHENG LIU1, XIN LIANG1, ZULI ZHOU2, HONGQING ZHUANG3, KUNKUN SUN4, YIMING WU5, JIN GU6 and WEI GUO1 1Musculoskeletal Tumor Center, 2Department of Thoracic Surgery, Peking University People's Hospital; 3Department of Radiotherapy, Peking University Third Hospital; 4Pathology Department, Peking University People's Hospital; 5Endocrinology Department, 6Department of Surgical Oncology, Peking University Shougang Hospital, Beijing 100044, P.R. China Received February 18, 2021; Accepted April 27, 2021 DOI: 10.3892/ol.2021.12813 Abstract. For osteosarcoma that progresses following median event‑free survival was 11.4 (IQR, 6.7‑18.4) months. first‑line chemotherapy, prognosis remains poor although The median overall survival time was 19.8 (IQR, 13.1‑30.6) anti‑angiogenesis tyrosine kinase inhibitors (TKIs) have been months. At the last follow‑up, 16/33 patients had tumour verified to prolong progression‑free survival. Apatinib has led downstaging, and all lesions had been completely resected. For to positive responses in the treatment of refractory osteosar‑ osteosarcoma with multiple sites of metastasis, apatinib + IE coma. However, it demonstrates only short‑lived activity, and demonstrated clinically meaningful antitumor activity and the disease control rate of musculoskeletal lesions is worse delayed disease progression in patients with recurrent or compared with that of pulmonary lesions. This treatment refractory osteosarcoma after failure of chemotherapy. This failure has been partly overcome by the addition of ifos‑ combination with manageable toxicity deserves further inves‑ famide and etoposide (IE). -

Apatinib Treatment in Metastatic Gastrointestinal Stromal Tumor
CASE REPORT published: 11 June 2019 doi: 10.3389/fonc.2019.00470 Apatinib Treatment in Metastatic Gastrointestinal Stromal Tumor Zhaolun Cai 1, Xin Chen 1, Bo Zhang 1 and Dan Cao 2* 1 Department of Gastrointestinal Surgery, West China Hospital, Sichuan University, Chengdu, China, 2 Department of Abdominal Oncology, Cancer Center of West China Hospital, Sichuan University, Chengdu, China Background: Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract. The clinical management of patients with metastatic GISTs is exceptionally challenging due to their poor prognosis. Apatinib is a multiple tyrosine kinase inhibitor. Here, we present the unique case with metastatic GISTs who derived clinical benefit from apatinib following the failure of imatinib and sunitinib. Case presentation: A 57-year-old man was admitted to our hospital diagnosed with metastatic and recurrent GISTs following surgical resection. Fifty-four months after the Edited by: first-line imatinib treatment, he developed progressive disease and then was treated Matiullah Khan, Asian Institute of Medicine, Science with cytoreductive surgery combined with imatinib. Disease progression occurred after 7 and Technology (AIMST) months. He then received second-line sunitinib and achieved a progression-free survival University, Malaysia of 11 months. Apatinib mesylate was then administered. Follow-up imaging revealed a Reviewed by: Sara Gandolfi, stable disease. Progression-free survival following apatinib therapy was at least 8 months. Dana–Farber Cancer Institute, The only toxicities were hypertension and proteinuria, which were both controllable United States and well-tolerated. Christian Badr, Massachusetts General Hospital and Conclusions: Treatment with apatinib provides an additional option for the treatment Harvard Medical School, United States of patients with GISTs refractory to imatinib and sunitinib. -

KEYNOTE-059) Approval (KEYNOTE-590)
CG-1 KEYTRUDA® for Patients With Advanced Gastric Cancer Nageatte Ibrahim, MD Vice President, Oncology Clinical Research Merck & Co., Inc. CG-2 KEYTRUDA® Has Demonstrated Clinical Benefit Across Multiple Indications and Tumor Types 19 Traditional approvals 13 Original traditional approvals in 9 different tumor types • Melanoma, 2 in non-small cell lung cancer (NSCLC), 2 in Head and neck squamous cell carcinoma (HNSCC), classical Hodgkin lymphoma (cHL), 2 in urothelial carcinoma, MSI-H colorectal cancer, 2 in esophageal and gastroesophageal junction (GEJ) carcinoma, renal cell carcinoma, cutaneous squamous cell carcinoma 6 Original accelerated approvals converted upon verification of benefit • Melanoma, 1L and 2L NSCLC, HNSCC, 3L+ cHL, primary mediastinal large B-cell lymphoma 10 Accelerated Approvals 6 Ongoing confirmatory studies • MSI-H (tumor agnostic), TMB-H (tumor agnostic), cervical cancer, Merkel cell carcinoma, endometrial carcinoma, triple-negative breast cancer 4 Confirmatory studies did not meet primary endpoints • Gastric cancer, hepatocellular carcinoma, urothelial carcinoma, small cell lung cancera MSI-H=microsatellite instability high; TMB-H=tumor mutational burden high. a Indication withdrawn after consultation with FDA. CG-3 Regulatory Approvals for KEYTRUDA® Relevant for Today’s Discussion Gastric and gastroesophageal junction (GEJ) cancer June 2015: Orphan Drug Sep 2017: 3L+ gastric Mar 2021: 1L esophageal Designation in gastric cancer cancer, accelerated and GEJ cancer, traditional (KEYNOTE-012) approval (KEYNOTE-059) approval (KEYNOTE-590) 2015 2016 2017 2018 2019 2020 2021 May 2017: MSI-H/dMMR June 2020: TMB-H accelerated approval accelerated approval MSI-H or MMR deficient solid tumors (2L+) TMB-H (≥ 10 mut/Mb) solid tumors (2L+) Tumor agnostic Mb=megabase; MMR=mismatch repair; mut=mutation; MSI-H=microsatellite instability high; TMB-H=tumor mutational burden high. -

The Efficacy and Safety of Apatinib Treatment for Patients With
Journal of Cancer 2018, Vol. 9 2773 Ivyspring International Publisher Journal of Cancer 2018; 9(16): 2773-2777. doi: 10.7150/jca.26376 Research Paper The Efficacy and Safety of Apatinib Treatment for Patients with Unresectable or Relapsed Liver Cancer: a retrospective study Liu Zhen1, Chen Jiali1, Fang Yong1, Xufeng Han2, Pan Hongming1, Han Weidong1 1. Department of Medical Oncology, Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang, China. 2. Department of Internal Medicine, Yuyao Traditional Chinese Medicine Hospital, Yuyao, Zhejiang, China Corresponding authors: Weidong Han, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, 3# East Qinchun Road, Hangzhou, Zhejiang, China, 310016. Phone: +86-571-86006926, E-mail: [email protected]; Hongming Pan, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, 3# East Qinchun Road, Hangzhou, Zhejiang, China, 310016. Phone: +86-571-86006922, E-mail: [email protected]. © Ivyspring International Publisher. This is an open access article distributed under the terms of the Creative Commons Attribution (CC BY-NC) license (https://creativecommons.org/licenses/by-nc/4.0/). See http://ivyspring.com/terms for full terms and conditions. Received: 2018.03.29; Accepted: 2018.05.08; Published: 2018.07.16 Abstract Purpose: Liver cancer is insensitive to chemotherapy. Sorafenib is currently the standard treatment for patients with advanced diseases, with mild survival extension and several intolerable drug-related side effects. The establishment of new treatments is an unmet clinical need. The aim of our study was to assess the efficacy and safety of apatinib, a novel antiangiogenic drug, in the treatment of patients with liver cancer. -

Bone Metastases from Gastric Cancer: What We Know and How to Deal with Them
Journal of Clinical Medicine Review Bone Metastases from Gastric Cancer: What We Know and How to Deal with Them Angelica Petrillo 1,2,* , Emilio Francesco Giunta 1,2 , Annalisa Pappalardo 1,2, Davide Bosso 1, Laura Attademo 1, Cinzia Cardalesi 1, Anna Diana 1,2 , Antonietta Fabbrocini 1, Teresa Fabozzi 1, Pasqualina Giordano 1, Margaret Ottaviano 1,3,4 , Mario Rosanova 1, Antonia Silvestri 1, Piera Federico 1 and Bruno Daniele 1 1 Medical Oncology Unit, Ospedale del Mare, 80147 Naples, Italy; [email protected] (E.F.G.); [email protected] (A.P.); [email protected] (D.B.); [email protected] (L.A.); [email protected] (C.C.); [email protected] (A.D.); [email protected] (A.F.); [email protected] (T.F.); [email protected] (P.G.); [email protected] (M.O.); [email protected] (M.R.); [email protected] (A.S.); [email protected] (P.F.); [email protected] (B.D.) 2 Department of Precision Medicine, School of Medicine, University of Study of Campania “L. Vanvitelli”, 80131 Naples, Italy 3 Department of Clinical Medicine and Surgery, University of Naples “Federico II”, 80131 Naples, Italy 4 CRCTR Rare Tumors Reference Center of Campania Region, 80131 Naples, Italy * Correspondence: [email protected] Abstract: Gastric cancer (GC) is the third cause of cancer-related death worldwide; the prognosis is poor especially in the case of metastatic disease. Liver, lymph nodes, peritoneum, and lung are the most frequent sites of metastases from GC; however, bone metastases from GC have been reported Citation: Petrillo, A.; Giunta, E.F.; in the literature. -

Apatinib Plus Camrelizumab (Anti-PD1 Therapy, SHR-1210)
Open access Original research J Immunother Cancer: first published as 10.1136/jitc-2020-000798 on 5 May 2020. Downloaded from Apatinib plus camrelizumab (anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO) progressing after chemotherapy: a single- arm, open- label, phase 2 trial 1 1 1 1 2 1 1 Lu Xie, Jie Xu, Xin Sun, Wei Guo , Jin Gu, Kuisheng Liu, Bingxin Zheng, Tingting Ren,1 Yi Huang,1 Xiaodong Tang,1 Taiqiang Yan,1 Rongli Yang,1 Kunkun Sun,3 Danhua Shen,3 Yuan Li4 To cite: Xie L, Xu J, Sun X, et al. ABSTRACT BACKGROUND Apatinib plus camrelizumab Background Results of our previous study showed high Osteosarcoma, a highly heterogeneous tumor (anti- PD1 therapy, SHR-1210) objective response but short- term activity of apatinib in for advanced osteosarcoma arising from mesenchymal tissues, is highly (APFAO) progressing after advanced osteosarcoma. We aimed to investigate the invasive and prone to hematogenous metas- chemotherapy: a single- arm, activity of apatinib in combination with camrelizumab tasis in the early stage with a 5- year overall open- label, phase 2 trial. in patients with inoperable high- grade osteosarcoma survival (OS) of 71% (95% CI 68% to 73%).1 Journal for ImmunoTherapy progressing after chemotherapy. However, after failure of chemotherapy of Cancer 2020;8:e000798. Methods This open- label, phase 2 trial was conducted doi:10.1136/jitc-2020-000798 including high- dose methotrexate (HD- at Peking University People’s Hospital. We enrolled MTX), doxorubicin (ADM), cisplatin (DDP), patients with advanced osteosarcoma progressed after ► Additional material is and ifosfamide (IFO), the treatment options chemotherapy. -
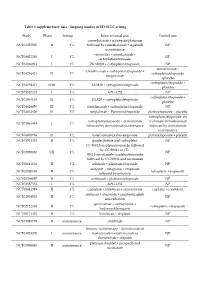
Ongoing Studies in ED-SCLC Setting Study Phase Setting
Table 1 supplementary data : Ongoing studies in ED-SCLC setting Study Phase Setting Interventional arm Control arm camrelizumab + irinotecan/platinum NCT04453930 II 1°L followed by camrelizumab + Apatinib NP maintenance venetoclax + atezolizumab + NCT04422210 I 1°L NP carboplatin/etoposide. NCT04346914 I 1°L ZKAB001 + carboplatin/etoposide NP atezolizumab + Atezolizumab + carboplatin/etoposide + NCT04256421 III 1°L carboplatin/etoposide tiragolumab +placebo carboplatin/etoposide + NCT04254471 II/III 1°L AL3810 + carboplatin/etoposide placebo NCT03387332 I 1°L APG-1252 NP carboplatin/etoposide + NCT04063163 III 1°L HLX10 + carboplatin/etoposide placebo NCT04028050 III 1°L atezolizumab + carboplatin/etoposide NP NCT04012606 III 1°L toripalimab + Platinum/etoposide platin/etoposide + placebo carboplatin/etoposide+du carboplatin/etoposide + durvalumab rvalumab+tremelimumab NCT03963414 I 1°L followed by durvalumab maintenance followed by durvalumab maintenance NCT04005716 III 1°L tislelizumab+platin+etoposide platin/etoposide + placebo NCT03913455 II 1°L guadecitabine and carboplatin NP CC-90011+cisplatin/etoposide followed by CC-90011 or CC- NCT03850067 I/II 1°L NP 90011+nivolumab+cisplatin/etoposide followed by CC-90011 and nivolumab NCT03841136 II 1°L anlotinib + platinum/etoposide NP anlotinib + lobaplatin + etoposide NCT03700359 II 1°L lobaplatin + etoposide followed by anlotinib NCT03568097 II 1°L avelumab + platinum/etoposide NP NCT03387332 I 1°L APG-1252 NP NCT01441349 II 1°L cisplatine + irinotecan + simvastatine cisplatin + irinotecan -

Apatinib in Patients with Relapsed Or Refractory Diffuse Large B Cell Lymphoma: a Phase II, Open-Label, Single-Arm, Prospective Study
Drug Design, Development and Therapy Dovepress open access to scientific and medical research Open Access Full Text Article CLINICAL TRIAL REPORT Apatinib in Patients with Relapsed or Refractory Diffuse Large B Cell Lymphoma: A Phase II, Open-Label, Single-Arm, Prospective Study This article was published in the following Dove Press journal: Drug Design, Development and Therapy Xinran Ma * Purpose: Treatment options for relapsed or refractory diffuse large B-cell lymphoma (RR Ling Li* DLBCL) represent an unmet medical need. Apatinib is a new oral tyrosine kinase inhibitor Lei Zhang mainly targeting vascular endothelial growth factor receptor-2 (VEGFR-2) to inhibit tumour fi Xiaorui Fu angiogenesis. In the present study, we evaluated the ef cacy and safety of apatinib for Xin Li patients with RR DLBCL. Patients and Methods: In this phase II, open-label, single-arm, prospective study, we Xinhua Wang enrolled patients aged 14–70 years with treatment failure of at least two chemotherapeutic Jingjing Wu regimens using Simon’s two-stage design. All patients were administered apatinib at an Zhenchang Sun initial dose of 500 mg on a 4-week cycle at home and visited the outpatient clinic every two Xudong Zhang cycles to evaluate efficacy and to record adverse events. We considered objective response Xiaoyan Feng rate (ORR) as the primary end point, and progression-free survival (PFS), and overall Yu Chang survival (OS) plus duration of response (DoR) as the secondary end point. (This trial was Zhiyuan Zhou registered at ClinicalTrials.gov, identifier: NCT03376958.). Feifei Nan Results: From January 2017 to February 2019, we screened 35 patients and enrolled 32 Jieming Zhang eligible patients. -

Apatinib Preferentially Inhibits PC9 Gefitinib-Resistant Cancer Cells By
Song et al. Cancer Cell Int (2019) 19:117 https://doi.org/10.1186/s12935-019-0836-8 Cancer Cell International PRIMARY RESEARCH Open Access Apatinib preferentially inhibits PC9 geftinib-resistant cancer cells by inducing cell cycle arrest and inhibiting VEGFR signaling pathway Yong‑An Song1†, Ting Ma2,3†, Xue‑Yan Zhang2,3, Xiang‑Song Cheng1, Ayobami‑Matthew Olajuyin1, Zhi‑Fu Sun4* and Xiao‑Ju Zhang1* Abstract Background: Lung cancer is one of the most common and deadly tumors around the world. Targeted therapy for patients with certain mutations, especially by use of tyrosine kinase inhibitors (TKIs) targeting epidermal growth factor receptor (EGFR), has provided signifcant beneft to patients. However, gradually developed resistance to the therapy becomes a major challenge in clinical practice and an alternative to treat such patients is needed. Herein, we report that apatinib, a novel anti‑angiogenic drug, efectively inhibits obtained geftinib‑resistant cancer cells but has no much efect on their parental sensitive cells. Methods: Geftinib‑resistant lung cancer cell line (PC9GR) was established from its parental sensitive line (PC9) with a traditional EGFR mutation after long time exposure to geftinib. Diferent concentrations of apatinib were used to treat PC9, PC9GR, and other two lung cancer cell lines for its anti‑growth efects. RNA sequencing was performed on PC9, PC9GR, and both after apatinib treatment to detect diferentially expressed genes and involved pathways. Protein expression of key cycle regulators p57, p27, CDK2, cyclin E2, and pRb was detected using Western blot. Xenograft mouse model was used to assess the anti‑tumor activity of apatinib in vivo. -

Pyrotinib for Metastatic Breast Cancer After Multi-Anti-HER2 Targeted Therapy: a Case Report
5 Case Report Page 1 of 5 Pyrotinib for metastatic breast cancer after multi-anti-HER2 targeted therapy: a case report Feng Li1,2, Li Bian1, Zerui Qu1, Jianbin Li1,3, Fengrui Xu1,2, Zefei Jiang1 1Department of Breast Oncology, The Fifth Medical Center of Chinese PLA General Hospital, Beijing 100071, China; 2Department of Breast Oncology, Academy of Military Medical Sciences, Beijing 100089, China; 3Medical Molecular Biology, Beijing Institute of Biotechnology, Beijing 100071, China Correspondence to: Li Bian. Department of Breast Oncology, The Fifth Medical Center of Chinese PLA General Hospital, No. 8 East Street, Fengtai District, Beijing 100071, China. Email: [email protected]. Abstract: We report a typical case of human epidermal growth factor receptor-2 (HER2)-positive breast cancer with multiple metastases after multi-anti-HER2 therapy which responded to afterwards pyrotinib and gained significantly longer progression free survival compared with other cases. A 41 years-old woman was diagnosed with HER2-positive breast cancer in 2011 and developed liver, lung and bone metastases in 2014 after neoadjuvant therapy, radical mastectomy, adjuvant chemotherapy and targeted therapy. She then received multi-anti-HER2 therapy with trastuzumab, lapatinib and T-DM 1. The patient later got brain metastases and received local resection. In the end, the patient received pyrotinib 400 mg. She then reached partial response and lung metastases were significantly improved. The patient was then followed-up and achieved 29 months progression free survival. We also have applied next generation sequencing (NGS) to demonstrate the efficacy of anti-HER2 treatment. This case provides a reference for future treatment of refractory breast cancer. -
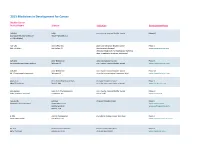
Adis R&D Insight
2015 Medicines in Development for Cancer Bladder Cancer Product Name Sponsor Indication Development Phase ABI-009 AADi non-muscle invasive bladder cancer Phase I/II (nanoparticle albumin-bound Pacific Palisades, CA mTOR inhibitor) ACP-196 Acerta Pharma platinum-refractory bladder cancer Phase II (Btk inhibitor) San Carlos, CA (combination therapy) www.acerta-pharma.com (see also head/neck, hematological, leukemia, lung, lymphoma, myeloma, pancreatic) ALT-801 Altor BioScience advanced bladder cancer, Phase II (immunotherapy fusion protein) Miramar, FL non-muscle invasive bladder cancer www.altorbioscience.com ALT-803 Altor BioScience non-muscle invasive bladder cancer Phase I/II (IL-15 superagonist complex) Miramar, FL (see also hematological, myeloma, skin) www.altorbioscience.com apatorsen OncoGenex Pharmaceuticals metastatic bladder cancer Phase II (Hsp27 inhibitor) Bothell, WA (see also lung, pancreatic, prostate) www.oncogenex.com apaziquone Spectrum Pharmaceuticals non-muscle invasive bladder cancer Phase III (DNA synthesis inhibitor) Henderson, NV (Fast Track) www.sppirx.com ASG-15ME Agensys relapsed bladder cancer Phase I (antibody drug conjugate) Santa Monica, CA www.agensys.com Seattle Genetics www.seattlegenetics.com Bothell, WA B-701 BioClin Therapeutics metastatic bladder cancer (2nd-line) Phase II (anti-FGFR3 mAb) San Ramon, CA www.bioclintherapeutics.com BC-819 BioCancell Therapeutics bladder cancer (2nd-line) Phase II (gene therapy) Jerusalem, Israel (see also pancreatic) www.biocancell.com Bladder Cancer Product Name Sponsor