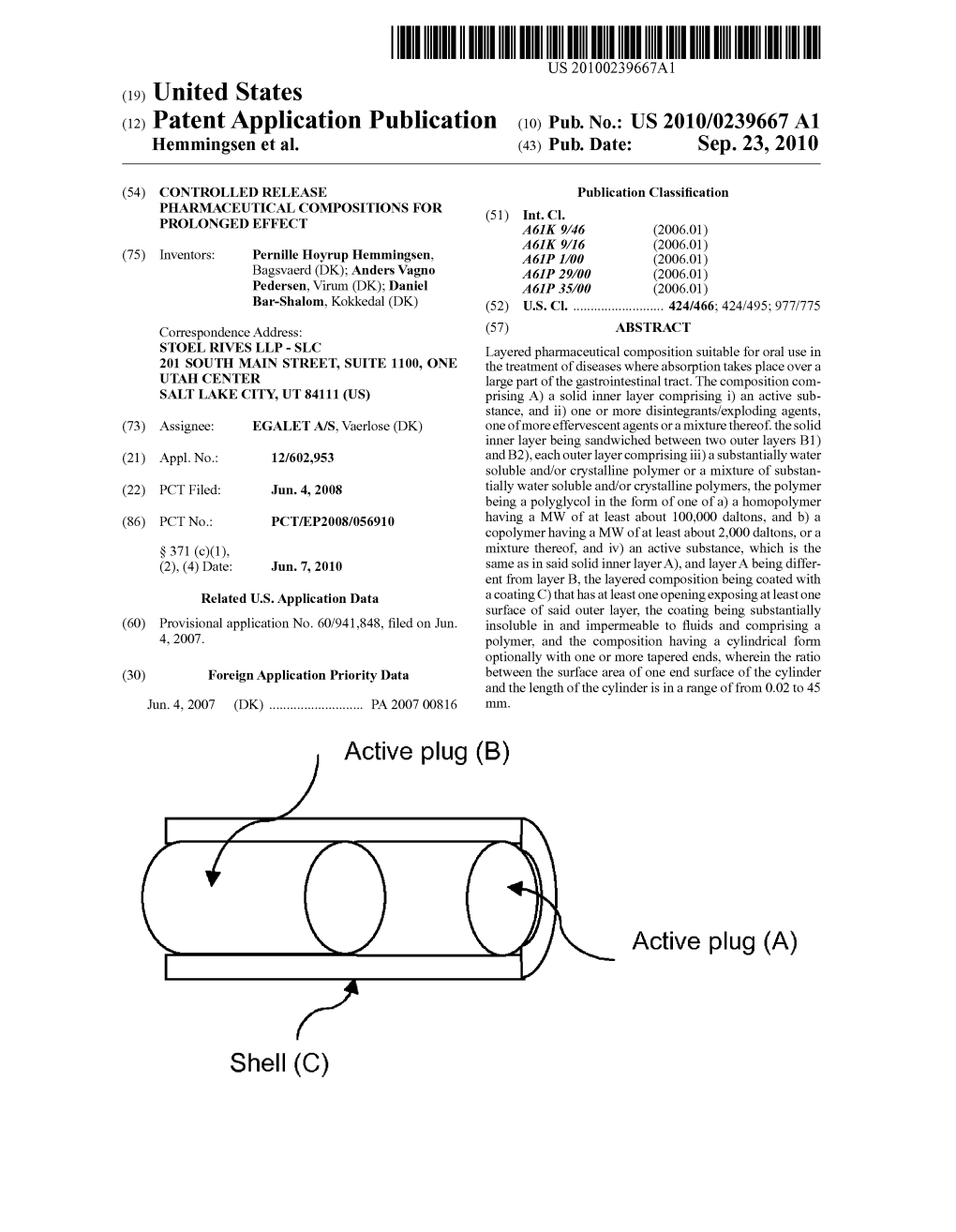
Active Plug (B) Shell (C) Active Plug
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WO 2015/179249 Al 26 November 2015 (26.11.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/179249 Al 26 November 2015 (26.11.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/11 (2006.01) A61K 38/08 (2006.01) kind of national protection available): AE, AG, AL, AM, C12N 15/00 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US2015/031213 HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (22) International Filing Date: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, 15 May 2015 (15.05.2015) MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (25) Filing Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (26) Publication Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 62/000,43 1 19 May 2014 (19.05.2014) US kind of regional protection available): ARIPO (BW, GH, 62/129,746 6 March 2015 (06.03.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (72) Inventors; and TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicants : GELLER, Bruce, L. -

(12) United States Patent (10) Patent No.: US 9,662.400 B2 Smith Et Al
USOO9662400B2 (12) United States Patent (10) Patent No.: US 9,662.400 B2 Smith et al. (45) Date of Patent: *May 30, 2017 (54) METHODS FOR PRODUCING A (2013.01); C08B 37/003 (2013.01); C08L 5/08 BODEGRADABLE CHITOSAN (2013.01); A6 IK 38/00 (2013.01); A61 L COMPOSITION AND USES THEREOF 2300/404 (2013.01) (58) Field of Classification Search (71) Applicant: University of Memphis Research CPC ...... A61K 47/36; A61K 31/00; A61K 9/7007; Foundation, Memphis, TN (US) A61K 9/0024; A61 L 15/28: A61L 27/20; A61L 27/58: A61L 31/042; C08B 37/003 (72) Inventors: James Keaton Smith, Memphis, TN USPC ................................ 514/23, 40, 777; 536/20 (US); Ashley C. Parker, Memphis, TN See application file for complete search history. (US); Jessica A. Jennings, Memphis, (56) References Cited TN (US); Benjamin T. Reves, Memphis, TN (US); Warren O. U.S. PATENT DOCUMENTS Haggard, Bartlett, TN (US) 4,895,724. A * 1/1990 Cardinal .............. A61K9/0024 424,278.1 (73) Assignee: The University of Memphis Research 5,541,233 A 7/1996 Roenigk Foundation, Memphis, TN (US) 5,958,443 A 9/1999 Viegas et al. 6,699,287 B2 3/2004 Son et al. (*) Notice: Subject to any disclaimer, the term of this 6,989,157 B2 1/2006 Gillis et al. patent is extended or adjusted under 35 7,371.403 B2 5/2008 McCarthy et al. 2003, OO15825 A1 1/2003 Sugie et al. U.S.C. 154(b) by 0 days. 2003/0206958 A1 11/2003 Cattaneo et al. -

Second and Third Generation Oral Fluoroquinolones
Therapeutic Class Overview Second and Third Generation Oral Fluoroquinolones Therapeutic Class • Overview/Summary: The second and third generation quinolones are approved to treat a variety of infections, including dermatologic, gastrointestinal, genitourinary, respiratory, as well as several miscellaneous infections.1-10 They are broad-spectrum agents that directly inhibit bacterial deoxyribonucleic acid (DNA) synthesis by blocking the actions of DNA gyrase and topoisomerase IV, which leads to bacterial cell death.11,12 The quinolones are most active against gram-negative bacilli and gram-negative cocci.12 Ciprofloxacin has the most potent activity against gram-negative bacteria. Norfloxacin, ciprofloxacin and ofloxacin have limited activity against streptococci and many anaerobes while levofloxacin and moxifloxacin have greater potency against gram-positive cocci, and moxifloxacin has enhanced activity against anaerobic bacteria.11-12 Gemifloxacin, levofloxacin and moxifloxacin are considered respiratory fluoroquinolones. They possess enhanced activity against Streptococcus pneumoniae while maintaining efficacy against Haemophilus influenzae, Moraxella catarrhalis and atypical pathogens. Resistance to the quinolones is increasing and cross-resistance among the various agents has been documented. Two mechanisms of bacterial resistance have been identified. These include mutations in chromosomal genes (DNA gyrase and/or topoisomerase IV) and altered drug permeability across the bacterial cell membranes.11-12 Clinical Guidelines support -

A TWO-YEAR RETROSPECTIVE ANALYSIS of ADVERSE DRUG REACTIONS with 5PSQ-031 FLUOROQUINOLONE and QUINOLONE ANTIBIOTICS 24Th Congress Of
A TWO-YEAR RETROSPECTIVE ANALYSIS OF ADVERSE DRUG REACTIONS WITH 5PSQ-031 FLUOROQUINOLONE AND QUINOLONE ANTIBIOTICS 24th Congress of V. Borsi1, M. Del Lungo2, L. Giovannetti1, M.G. Lai1, M. Parrilli1 1 Azienda USL Toscana Centro, Pharmacovigilance Centre, Florence, Italy 2 Dept. of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA), 27-29 March 2019 Section of Pharmacology and Toxicology , University of Florence, Italy BACKGROUND PURPOSE On 9 February 2017, the Pharmacovigilance Risk Assessment Committee (PRAC) initiated a review1 of disabling To review the adverse drugs and potentially long-lasting side effects reported with systemic and inhaled quinolone and fluoroquinolone reactions (ADRs) of antibiotics at the request of the German medicines authority (BfArM) following reports of long-lasting side effects systemic and inhaled in the national safety database and the published literature. fluoroquinolone and quinolone antibiotics that MATERIAL AND METHODS involved peripheral and central nervous system, Retrospective analysis of ADRs reported in our APVD involving ciprofloxacin, flumequine, levofloxacin, tendons, muscles and joints lomefloxacin, moxifloxacin, norfloxacin, ofloxacin, pefloxacin, prulifloxacin, rufloxacin, cinoxacin, nalidixic acid, reported from our pipemidic given systemically (by mouth or injection). The period considered is September 2016 to September Pharmacovigilance 2018. Department (PVD). RESULTS 22 ADRs were reported in our PVD involving fluoroquinolone and quinolone antibiotics in the period considered and that affected peripheral or central nervous system, tendons, muscles and joints. The mean patient age was 67,3 years (range: 17-92 years). 63,7% of the ADRs reported were serious, of which 22,7% caused hospitalization and 4,5% caused persistent/severe disability. 81,8% of the ADRs were reported by a healthcare professional (physician, pharmacist or other) and 18,2% by patient or a non-healthcare professional. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

The Effect of Chloramphenicol on BB88 Murine Erythroleukemia Cells
Western Michigan University ScholarWorks at WMU Dissertations Graduate College 8-2007 The Effect of Chloramphenicol on BB88 Murine Erythroleukemia Cells Peter K. W. Harris Western Michigan University Follow this and additional works at: https://scholarworks.wmich.edu/dissertations Part of the Chemistry Commons Recommended Citation Harris, Peter K. W., "The Effect of Chloramphenicol on BB88 Murine Erythroleukemia Cells" (2007). Dissertations. 872. https://scholarworks.wmich.edu/dissertations/872 This Dissertation-Open Access is brought to you for free and open access by the Graduate College at ScholarWorks at WMU. It has been accepted for inclusion in Dissertations by an authorized administrator of ScholarWorks at WMU. For more information, please contact [email protected]. THE EFFECT OF CHLORAMPHENICOL ON BB88 MURINE ERYTHROLEUKEMIA CELLS by Peter K. W. Harris A Dissertation Submitted to the Faculty o f The Graduate College in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Department of Biological Sciences Western Michigan University Kalamazoo, Michigan August 2007 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. THE EFFECT OF CHLORAMPHENICOL ON BB88 MURINE ERYTHROLEUKEMIA CELLS Peter K. W. Harris, Ph.D. Western Michigan University, 2007 DNA microarrays can be used to measure genome-wide transcript levels. These measurements may be useful in understanding cellular changes induced by a chemical agent. In this study, Affymetrix microarray technology has been used to study the effects of chloramphenicol, an antibiotic that inhibits bacterial and mitochondrial protein synthesis, on the transcription profile in mammalian cells. Transcript levels in BB88 murine erythroleukemia cells treated with 50 micromolar (pM) chloramphenicol, a concentration shown to inhibit BB 88 proliferation, are measured. -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

AMEG Categorisation of Antibiotics
12 December 2019 EMA/CVMP/CHMP/682198/2017 Committee for Medicinal Products for Veterinary use (CVMP) Committee for Medicinal Products for Human Use (CHMP) Categorisation of antibiotics in the European Union Answer to the request from the European Commission for updating the scientific advice on the impact on public health and animal health of the use of antibiotics in animals Agreed by the Antimicrobial Advice ad hoc Expert Group (AMEG) 29 October 2018 Adopted by the CVMP for release for consultation 24 January 2019 Adopted by the CHMP for release for consultation 31 January 2019 Start of public consultation 5 February 2019 End of consultation (deadline for comments) 30 April 2019 Agreed by the Antimicrobial Advice ad hoc Expert Group (AMEG) 19 November 2019 Adopted by the CVMP 5 December 2019 Adopted by the CHMP 12 December 2019 Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2020. Reproduction is authorised provided the source is acknowledged. Categorisation of antibiotics in the European Union Table of Contents 1. Summary assessment and recommendations .......................................... 3 2. Introduction ............................................................................................ 7 2.1. Background ........................................................................................................ -

Food Sample Preparation for the Determination of Sulfonamides by High-Performance Liquid Chromatography: State-Of-The-Art
separations Review Food Sample Preparation for the Determination of Sulfonamides by High-Performance Liquid Chromatography: State-of-the-Art Dimitrios Bitas 1, Abuzar Kabir 2 ID , Marcello Locatelli 3 ID and Victoria Samanidou 1,* ID 1 Laboratory of Analytical Chemistry, Department of Chemistry, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece; [email protected] 2 International Forensic Research Institute, Department of Chemistry and Biochemistry, Florida International University, 11200 SW 8th St, Miami, FL 33199, USA; akabir@fiu.edu 3 Department of Pharmacy, University “G. d’Annunzio” of Chieti-Pescara, 66100 Chieti, Italy; [email protected] * Correspondence: [email protected]; Tel.: +30-231-099-7698 Received: 30 April 2018; Accepted: 28 May 2018; Published: 4 June 2018 Abstract: Antibiotics are a common practice in veterinary medicine, mainly for therapeutic purposes. Sectors of application include livestock farming, aquacultures, and bee-keeping, where bacterial infections are frequent and can be economically damaging. However, antibiotics are usually administered in sub-therapeutic doses as prophylactic and growth promoting agents. Due to their excessive use, antibiotic residues can be present in foods of animal origin, which include meat, fish, milk, eggs, and honey, posing health risks to consumers. For this reason, authorities have set maximum residue limits (MRLs) of certain antibiotics in food matrices, while analytical methods for their determination have been developed. This work focuses on antibiotic extraction and determination, part of which was presented at the “1st Conference in Chemistry for Graduate, Postgraduate Students and PhD Candidates at the Aristotle University of Thessaloniki”. Taking a step further, this paper is a review of the most recent sample preparation protocols applied for the extraction of sulfonamide antibiotics from food samples and their determination with high-performance liquid chromatography (HPLC), covering a five-year period. -

FLUOROQUINOLONES: from Structure to Activity and Toxicity
FLUOROQUINOLONES: from structure to activity and toxicity F. Van Bambeke, Pharm. D. & P. M. Tulkens, MD, PhD Unité de Pharmacologie Cellulaire et Moléculaire Université Catholique de Louvain, Brussels, Belgium SBIMC / BVIKM www.sbimc.org - www.bvikm.org www.md.ucl.ac.be/facm www.isap.org soon... Mechanism of action of fluoroquinolones: the basics... PORIN DNA Topo DNA gyrase isomerase Gram (-) Gram (+) 2 key enzymes in DNA replication: DNA gyrase topoisomerase IV bacterial DNA is supercoiled Ternary complex DNA - enzyme - fluoroquinolone DNA GYRASE catalytic subunits COVALENTLY CLOSED CIRCULAR DNA FLUOROQUINOLONES: DNA GYRASE ATP binding subunits 4 stacked molecules (Shen, in Quinolone Antimicrobial Agents, 1993) Resistance to fluoroquinolones: the basics decreased efflux pump permeability DNA mutation of DNA gyrase Topo isomerase the enzymes Gram (-) Gram (+) Fluoroquinolones are the first entirely man-made antibiotics: do we understand our molecule ? R5 O R COOH 6 R7 X8 N R1 Don’t panic, we will travel together…. Chemistry and Activity This is where all begins... The pharmacophore common to all fluoroquinolones BINDING TO DNA R5 O O R C 6 - BINDING TO O BINDING TO THE ENZYME THE ENZYME R7 X8 N R1 AUTO-ASSEMBLING DOMAIN (for stacking) From chloroquine to nalidixic acid... nalidixic acid N CH3 O O HN CH 3 C - O chloroquine CH N N Cl N 3 C2H5 1939 O O C O- 1962 Cl N 1958 C2H5 7-chloroquinoline (synthesis intermediate found to display antibacterial activity) Nalidixic acid * a • typical chemical features of O O fluoroquinolones (a, b, c) BUT a naphthridone C - O- b (N at position 8: ) H C N N 3 • limited usefulness as drug C H 2 5 • narrow antibacterial spectrum c (Enterobacteriaceae only) • short half-life (1.5h) • high protein binding (90%) * Belg. -

EMA/CVMP/158366/2019 Committee for Medicinal Products for Veterinary Use
Ref. Ares(2019)6843167 - 05/11/2019 31 October 2019 EMA/CVMP/158366/2019 Committee for Medicinal Products for Veterinary Use Advice on implementing measures under Article 37(4) of Regulation (EU) 2019/6 on veterinary medicinal products – Criteria for the designation of antimicrobials to be reserved for treatment of certain infections in humans Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2019. Reproduction is authorised provided the source is acknowledged. Introduction On 6 February 2019, the European Commission sent a request to the European Medicines Agency (EMA) for a report on the criteria for the designation of antimicrobials to be reserved for the treatment of certain infections in humans in order to preserve the efficacy of those antimicrobials. The Agency was requested to provide a report by 31 October 2019 containing recommendations to the Commission as to which criteria should be used to determine those antimicrobials to be reserved for treatment of certain infections in humans (this is also referred to as ‘criteria for designating antimicrobials for human use’, ‘restricting antimicrobials to human use’, or ‘reserved for human use only’). The Committee for Medicinal Products for Veterinary Use (CVMP) formed an expert group to prepare the scientific report. The group was composed of seven experts selected from the European network of experts, on the basis of recommendations from the national competent authorities, one expert nominated from European Food Safety Authority (EFSA), one expert nominated by European Centre for Disease Prevention and Control (ECDC), one expert with expertise on human infectious diseases, and two Agency staff members with expertise on development of antimicrobial resistance . -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01