Granulocyte-Colony Stimulating Factor Therapy to Induce Neovascularization in Ischemic Heart Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![About Pigs [PDF]](https://docslib.b-cdn.net/cover/0911/about-pigs-pdf-50911.webp)
About Pigs [PDF]
May 2015 About Pigs Pigs are highly intelligent, social animals, displaying elaborate maternal, communicative, and affiliative behavior. Wild and feral pigs inhabit wide tracts of the southern and mid-western United States, where they thrive in a variety of habitats. They form matriarchal social groups, sleep in communal nests, and maintain close family bonds into adulthood. Science has helped shed light on the depths of the remarkable cognitive abilities of pigs, and fosters a greater appreciation for these often maligned and misunderstood animals. Background Pigs—also called swine or hogs—belong to the Suidae family1 and along with cattle, sheep, goats, camels, deer, giraffes, and hippopotamuses, are part of the order Artiodactyla, or even-toed ungulates.2 Domesticated pigs are descendants of the wild boar (Sus scrofa),3,4 which originally ranged through North Africa, Asia and Europe.5 Pigs were first domesticated approximately 9,000 years ago.6 The wild boar became extinct in Britain in the 17th century as a result of hunting and habitat destruction, but they have since been reintroduced.7,8 Feral pigs (domesticated animals who have returned to a wild state) are now found worldwide in temperate and tropical regions such as Australia, New Zealand, and Indonesia and on island nations, 9 such as Hawaii.10 True wild pigs are not native to the New World.11 When Christopher Columbus landed in Cuba in 1493, he brought the first domestic pigs—pigs who subsequently spread throughout the Spanish West Indies (Caribbean).12 In 1539, Spanish explorers brought pigs to the mainland when they settled in Florida. -

ARTICLES Fibroblast Growth Factors 1, 2, 17, and 19 Are The
0031-3998/07/6103-0267 PEDIATRIC RESEARCH Vol. 61, No. 3, 2007 Copyright © 2007 International Pediatric Research Foundation, Inc. Printed in U.S.A. ARTICLES Fibroblast Growth Factors 1, 2, 17, and 19 Are the Predominant FGF Ligands Expressed in Human Fetal Growth Plate Cartilage PAVEL KREJCI, DEBORAH KRAKOW, PERTCHOUI B. MEKIKIAN, AND WILLIAM R. WILCOX Medical Genetics Institute [P.K., D.K., P.B.M., W.R.W.], Cedars-Sinai Medical Center, Los Angeles, California 90048; Department of Obstetrics and Gynecology [D.K.] and Department of Pediatrics [W.R.W.], UCLA School of Medicine, Los Angeles, California 90095 ABSTRACT: Fibroblast growth factors (FGF) regulate bone growth, (G380R) or TD (K650E) mutations (4–6). When expressed at but their expression in human cartilage is unclear. Here, we deter- physiologic levels, FGFR3-G380R required, like its wild-type mined the expression of entire FGF family in human fetal growth counterpart, ligand for activation (7). Similarly, in vitro cul- plate cartilage. Using reverse transcriptase PCR, the transcripts for tivated human TD chondrocytes as well as chondrocytes FGF1, 2, 5, 8–14, 16–19, and 21 were found. However, only FGF1, isolated from Fgfr3-K644M mice had an identical time course 2, 17, and 19 were detectable at the protein level. By immunohisto- of Fgfr3 activation compared with wild-type chondrocytes and chemistry, FGF17 and 19 were uniformly expressed within the showed no receptor activation in the absence of ligand (8,9). growth plate. In contrast, FGF1 was found only in proliferating and hypertrophic chondrocytes whereas FGF2 localized predominantly to Despite the importance of the FGF ligand for activation of the resting and proliferating cartilage. -

Disruption of Fibroblast Growth Factor Signal
Cancer Therapy: Preclinical Disruption of Fibroblast Growth Factor Signal Pathway Inhibits the Growth of Synovial Sarcomas: Potential Application of Signal Inhibitors to MolecularTarget Therapy Ta t s u y a I s hi b e , 1, 2 Tomitaka Nakayama,2 Ta k e s h i O k a m o t o, 1, 2 Tomoki Aoyama,1Koichi Nishijo,1, 2 Kotaro Roberts Shibata,1, 2 Ya s u ko Shim a ,1, 2 Satoshi Nagayama,3 Toyomasa Katagiri,4 Yusuke Nakamura, 4 Takashi Nakamura,2 andJunya Toguchida 1 Abstract Purpose: Synovial sarcoma is a soft tissue sarcoma, the growth regulatory mechanisms of which are unknown.We investigatedthe involvement of fibroblast growth factor (FGF) signals in synovial sarcoma andevaluatedthe therapeutic effect of inhibiting the FGF signal. Experimental Design:The expression of 22 FGF and4 FGF receptor (FGFR) genes in18prima- ry tumors andfive cell lines of synovial sarcoma were analyzedby reverse transcription-PCR. Effects of recombinant FGF2, FGF8, andFGF18 for the activation of mitogen-activatedprotein kinase (MAPK) andthe growth of synovial sarcoma cell lines were analyzed.Growth inhibitory effects of FGFR inhibitors on synovial sarcoma cell lines were investigated in vitro and in vivo. Results: Synovial sarcoma cell lines expressedmultiple FGF genes especially those expressed in neural tissues, among which FGF8 showedgrowth stimulatory effects in all synovial sarcoma cell lines. FGF signals in synovial sarcoma induced the phosphorylation of extracellular signal ^ regulatedkinase (ERK1/2) andp38MAPK but not c-Jun NH 2-terminal kinase. Disruption of the FGF signaling pathway in synovial sarcoma by specific inhibitors of FGFR causedcell cycle ar- rest leading to significant growth inhibition both in vitro and in vivo.Growthinhibitionbythe FGFR inhibitor was associatedwith a down-regulation of phosphorylatedERK1/2 but not p38MAPK, andan ERK kinase inhibitor also showedgrowth inhibitory effects for synovial sar- coma, indicating that the growth stimulatory effect of FGF was transmitted through the ERK1/2. -

FGF Signaling Network in the Gastrointestinal Tract (Review)
163-168 1/6/06 16:12 Page 163 INTERNATIONAL JOURNAL OF ONCOLOGY 29: 163-168, 2006 163 FGF signaling network in the gastrointestinal tract (Review) MASUKO KATOH1 and MASARU KATOH2 1M&M Medical BioInformatics, Hongo 113-0033; 2Genetics and Cell Biology Section, National Cancer Center Research Institute, Tokyo 104-0045, Japan Received March 29, 2006; Accepted May 2, 2006 Abstract. Fibroblast growth factor (FGF) signals are trans- Contents duced through FGF receptors (FGFRs) and FRS2/FRS3- SHP2 (PTPN11)-GRB2 docking protein complex to SOS- 1. Introduction RAS-RAF-MAPKK-MAPK signaling cascade and GAB1/ 2. FGF family GAB2-PI3K-PDK-AKT/aPKC signaling cascade. The RAS~ 3. Regulation of FGF signaling by WNT MAPK signaling cascade is implicated in cell growth and 4. FGF signaling network in the stomach differentiation, the PI3K~AKT signaling cascade in cell 5. FGF signaling network in the colon survival and cell fate determination, and the PI3K~aPKC 6. Clinical application of FGF signaling cascade in cell polarity control. FGF18, FGF20 and 7. Clinical application of FGF signaling inhibitors SPRY4 are potent targets of the canonical WNT signaling 8. Perspectives pathway in the gastrointestinal tract. SPRY4 is the FGF signaling inhibitor functioning as negative feedback apparatus for the WNT/FGF-dependent epithelial proliferation. 1. Introduction Recombinant FGF7 and FGF20 proteins are applicable for treatment of chemotherapy/radiation-induced mucosal injury, Fibroblast growth factor (FGF) family proteins play key roles while recombinant FGF2 protein and FGF4 expression vector in growth and survival of stem cells during embryogenesis, are applicable for therapeutic angiogenesis. Helicobacter tissues regeneration, and carcinogenesis (1-4). -

Ryerson University Spring Graduates
Ryerson University Spring Graduates June 2020 Faculty of Arts 2 Faculty of Communication & Design 11 Faculty of Community Services 21 Faculty of Engineering and Architectural Science 35 Faculty of Science 46 Ted Rogers School of Management 54 Yeates School of Graduate Studies 71 The G. Raymond Chang School of Continuing Education 73 Faculty of Arts Pamela Sugiman Dean Faculty of Arts Janice Fukakusa Chancellor Mohamed Lachemi President and Vice-Chancellor Charmaine Hack Registrar Ryerson Gold Medal Presented to Mayah Obadia Geographic Analysis 2 Faculty of Arts Undergraduate Degree Programs Arts and Contemporary Studies Bachelor of Arts (Honours) *Diana Abo Harmouch Carmen Jajjo *Megumi Noteboom *Sima Rebecca Abrams Leya Jasat Valentina Padure Qeyam Amiri Sophie Johnson *Naiomi Marcia Perera Brodie Barrick Babina Kamalanathan Charlotte Jane Prokopec Rebecca Claire Chen Caroline Susan Kewley Regan Reynolds Erin Tanya Clarke Jessica Laurenza Joshua Ricci *Megan Lisa Devoe Claire Lowenstein Kaitlin Anganie Seepersaud *Manpreet Kaur Dhaliwal *Avigayil Margolis Gabriela Skwarko Tatum Lynn Donovan Sara McArthur Julia Macey Sullivan Faith Raha Giahi *Nadia Celeste McNairn *Helen Gillian Webb Meagan Gove *Mahbod Mehrvarz *Michael Worbanski Salem Habtom Andrew Moon Smyrna Wright *William Hanchar *Liana Gabriella Mortin Calum Jacques Potoula Mozas Criminology Bachelor of Arts (Honours) *Annabelle Adjei *Jenna Anne Giannini Veronica Hiu Lam Lee Stanislav Babinets Albina Glatman Karishma Catherine Lutchman Hela Bakhtari Farah Khaled Gregni Simbiat -

FY19 Annual Report View Report
Annual Report 2018–19 3 Introduction 5 Metropolitan Opera Board of Directors 6 Season Repertory and Events 14 Artist Roster 16 The Financial Results 20 Our Patrons On the cover: Yannick Nézet-Séguin takes a bow after his first official performance as Jeanette Lerman-Neubauer Music Director PHOTO: JONATHAN TICHLER / MET OPERA 2 Introduction The 2018–19 season was a historic one for the Metropolitan Opera. Not only did the company present more than 200 exiting performances, but we also welcomed Yannick Nézet-Séguin as the Met’s new Jeanette Lerman- Neubauer Music Director. Maestro Nézet-Séguin is only the third conductor to hold the title of Music Director since the company’s founding in 1883. I am also happy to report that the 2018–19 season marked the fifth year running in which the company’s finances were balanced or very nearly so, as we recorded a very small deficit of less than 1% of expenses. The season opened with the premiere of a new staging of Saint-Saëns’s epic Samson et Dalila and also included three other new productions, as well as three exhilarating full cycles of Wagner’s Ring and a full slate of 18 revivals. The Live in HD series of cinema transmissions brought opera to audiences around the world for the 13th season, with ten broadcasts reaching more than two million people. Combined earned revenue for the Met (box office, media, and presentations) totaled $121 million. As in past seasons, total paid attendance for the season in the opera house was 75%. The new productions in the 2018–19 season were the work of three distinguished directors, two having had previous successes at the Met and one making his company debut. -

The Role of Fibroblast Growth Factor Signalling in Echinococcus
bioRxiv preprint doi: https://doi.org/10.1101/457168; this version posted October 30, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 1 2 The role of fibroblast growth factor signalling in Echinococcus 3 multilocularis development and host-parasite interaction 4 5 Sabine Förster1, Uriel Koziol1,2, Tina Schäfer1, Raphael Duvoisin1, Katia Cailliau3, Mathieu 6 Vanderstraete4, Colette Dissous4, and Klaus Brehm1* 7 8 1University of Würzburg, Institute of Hygiene and Microbiology, Josef-Schneider-Strasse 2, 9 D-97080 Würzburg, Germany 10 2Universidad de la República, Facultad de Ciencias, Sección Biología Celular, Montevideo, 11 Uruguay 12 3CNRS UMR 8576, University of Lille, 59650, Villeneuve d’Asq, France 13 4Center for Infection and Immunology of Lille, Inserm U1019, CNRS-UMR 8204, University 14 of Lille, Lille, France 15 16 Short title: Host FGF stimulates Echinococcus larval development 17 18 *Corresponding author. 19 Email: [email protected] (KB) 20 bioRxiv preprint doi: https://doi.org/10.1101/457168; this version posted October 30, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 2 21 Abstract 22 Background: Alveolar echinococcosis (AE) is a lethal zoonosis caused by the metacestode 23 larva of the tapeworm Echinococcus multilocularis. -

Pig Husbandry in Iron Age Israel and Judah
Pig Husbandry in Iron Age Israel and Judah: New Insights Regarding the Origin of the "Taboo" Author(s): Lidar Sapir-Hen, Guy Bar-Oz, Yuval Gadot and Israel Finkelstein Source: Zeitschrift des Deutschen Palästina-Vereins (1953-), Bd. 129, H. 1 (2013), pp. 1-20 Published by: Deutscher verein zur Erforschung Palästinas Stable URL: https://www.jstor.org/stable/43664894 Accessed: 03-10-2018 18:50 UTC JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at https://about.jstor.org/terms Deutscher verein zur Erforschung Palästinas is collaborating with JSTOR to digitize, preserve and extend access to Zeitschrift des Deutschen Palästina-Vereins (1953-) This content downloaded from 129.2.19.102 on Wed, 03 Oct 2018 18:50:48 UTC All use subject to https://about.jstor.org/terms Pig Husbandry in Iron Age Israel and Judah New Insights Regarding the Origin of the "Taboo" By Lidar Sapir-Hen, Guy Bar-Oz, Yuval Gadot, and Israel Finkelstein 1. Introduction The biblical prohibition against the consumption of pork (Lev 11:7; Deut 14:8), observed in Judaism for over two millennia, is the reason for the special attention paid to the appearance of pig bones in Iron Age strata in the southern Levant1. -
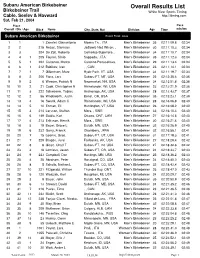
Overall Results List
Subaru American Birkebeiner Overall Results List Birkebeiner Trail White River Sports Timing Cable, Seeley & Hayward http://itiming.com Sat, Feb 21, 2004 Pl ace Pace Overall / Div / Age Bib # Name City, State, Nat Division Age Time min/km Subaru American Birkebeiner Event Field: 3833 1 1 1 Zanetel, Gianantonio Moena Tn, , ITA Men's Birkebeiner 35 02:11:09.8 02:34 2 2 215 Rezac, Stanislav Jadlowic Nad Wison, , Men's Birkebeiner 30 02:11:10.3 02:34 3 3 204 De Zolt, Roberto CoZmEelico Superiore, , Men's Birkebeiner 34 02:11:10.7 02:34 4 4 1 205 Fauner, Silvio SITaAppada, , ITA Men's Birkebeiner 35 02:11:12.3 02:34 5 5 1 202 Cattaneo, Marco Caronno Pertusellava, , Men's Birkebeiner 29 02:11:13.4 02:34 6 6 1 212 Babikov, Ivan ,I T, ACAN Men's Birkebeiner 23 02:11:14.1 02:34 7 7 1 7 Gilbertson, Marc Hyde Park, VT, USA Men's Birkebeiner 34 02:11:19.7 02:34 8 8 2 206 Flora, Lars Subaru FT, MT, USA Men's Birkebeiner 26 02:12:35.4 02:35 9 9 2 6 Weaver, Patrick N Newmarket, NH, USA Men's Birkebeiner 34 02:12:51.8 02:36 10 10 2 21 Cook, Christopher R Rhinelander, WI, USA Men's Birkebeiner 23 02:13:21.9 02:36 11 11 3 222 Schwoerer, Tobias Anchorage, AK, USA Men's Birkebeiner 28 02:13:43.7 02:37 12 12 2 56 Wadsworth, Justin Bend , OR, USA Men's Birkebeiner 35 02:15:23.1 02:39 13 13 4 16 Swank, Adam C Rhinelander, WI, USA Men's Birkebeiner 28 02:16:06.8 02:40 14 14 5 10 Enman, Eli Huntington, VT, USA Men's Birkebeiner 26 02:16:08.2 02:40 15 15 3 214 Larsson, Staffan Mora, , SWE Men's Birkebeiner 33 02:16:10.4 02:40 16 16 6 149 Saidla, Karl Ottawa, ONT, -

The Food Industry Scorecard
THE FOOD INDUSTRY SCORECARD An evaluation of food companies’ progress making—and keeping— animal welfare promises humanesociety.org/scorecard Executive summary Most of the largest U.S. food companies have publicly pledged to eliminate certain animal abuses from their supply chains. But as countless consumers have asked: are they keeping their promises? For context, the vast majority of animals in our food system live Here’s the good news: that kind of radical view is out of in dismal conditions. Mother pigs are locked in gestation crates step with traditional American values. Agribusiness may see ani- so small they can’t turn around. Egg-laying hens are crammed mals as mere machines, but consumers don’t. into cages so tightly they can’t even spread their wings. And chickens in the poultry industry are bred to grow so large, so ɠ As the American Farm Bureau reports, nearly all consumers (95%) believe farm animals should be fast they suffer from agonizing leg disorders. treated well. It wasn’t always this way. Throughout history, animals hav- en’t been forced to endure such miserable lives. (And today, ɠ The Food Marketing Institute found that animal welfare is shoppers’ second most important social issue. there are certainly farmers who don’t use these abusive prac- tices.) But as agri-culture developed into agri-business, the ɠ The food industry analytics firm Technomic concluded industry’s relationship to animals became more severe. that for American restaurant patrons, concerns about animal cruelty outweigh those regarding the “Forget the pig is an animal,” urged Hog Farm Management environment, fair trade, local sourcing and other issues. -

Fall 2021 Kids OMNIBUS
FALL 2021 RAINCOAST OMNIBUS Kids This edition of the catalogue was printed on May 13, 2021. To view updates, please see the Fall 2021 Raincoast eCatalogue or visit www.raincoast.com Raincoast Books Fall 2021 - Kids Omnibus Page 1 of 266 A Cub Story by Alison Farrell and Kristen Tracy Timeless and nostalgic, quirky and fresh, lightly educational and wholly heartfelt, this autobiography of a bear cub will delight all cuddlers and snugglers. See the world through a bear cub's eyes in this charming book about finding your place in the world. Little cub measures himself up to the other animals in the forest. Compared to a rabbit, he is big. Compared to a chipmunk, he is HUGE. Compared to his mother, he is still a little cub. The first in a series of board books pairs Kristen Tracy's timeless and nostalgic text with Alison Farrell's sweet, endearing art for an adorable treatment of everyone's favorite topic, baby animals. Author Bio Chronicle Books Alison Farrell has a deep and abiding love for wild berries and other foraged On Sale: Sep 28/21 foods. She lives, bikes, and hikes in Portland, Oregon, and other places in the 6 x 9 • 22 pages Pacific Northwest. full-color illustrations throughout 9781452174587 • $14.99 Kristen Tracy writes books for teens and tweens and people younger than Juvenile Fiction / Animals / Baby Animals • Ages 2-4 that, and also writes poetry for adults. She's spent a lot of her life teaching years writing at places like Johnson State College, Western Michigan University, Brigham Young University, 826 Valencia, and Stanford University. -

Downloaded from Bioscientifica.Com at 09/30/2021 03:40:24AM Via Free Access
27 7 Endocrine-Related E Labanca et al. FGF axis in bone metastasis 27:7 R255–R265 Cancer REVIEW Fibroblast growth factors signaling in bone metastasis Estefania Labanca1, Elba S Vazquez2,3, Paul G Corn1, Justin M Roberts1, Fen Wang4, Christopher J Logothetis1 and Nora M Navone1 1Department of Genitourinary Medical Oncology and the David H. Koch Center for Applied Research of Genitourinary Cancers, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA 2Laboratorio de Inflamación y Cáncer, Departamento de Química Biológica, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Buenos Aires, Argentina 3CONICET – Universidad de Buenos Aires, Instituto de Química Biológica de la Facultad de Ciencias Exactas y Naturales (IQUIBICEN), Buenos Aires, Argentina 4Institute of Biosciences and Technology, Texas A&M Health Science Center, Houston, Texas, USA Correspondence should be addressed to N M Navone: [email protected] Abstract Many solid tumors metastasize to bone, but only prostate cancer has bone as a Key Words single, dominant metastatic site. Recently, the FGF axis has been implicated in cancer f prostate cancer progression in some tumors and mounting evidence indicate that it mediates prostate f bone metastasis cancer bone metastases. The FGF axis has an important role in bone biology and f fibroblast growth factors mediates cell-to-cell communication. Therefore, we discuss here basic concepts of f fibroblast growth factor bone biology, FGF signaling axis, and FGF axis function in adult bone, to integrate these receptors concepts in our current understanding of the role of FGF axis in bone metastases. Endocrine-Related Cancer (2020) 27, R255–R265 Introduction Development of metastases is a complex and demanding cancer progression.