Diffuse Large B-Cell Lymphoma Presenting As a Sacral Tumor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

From Sacrum to Spine: a Complex Case of Sacrococcygeal Teratoma
From Sacrum to Spine: A Complex Case of Sacrococcygeal Teratoma Jennifer Young, MN, RN(EC) NP-Pediatrics, NNP-BC Hazel Pleasants-Terashita, MN, RN(EC) NP-Pediatrics, NNP-BC Continuing Nursing Education (CNE) ABSTRACT Credit The test questions are pro- Neonatal tumors occur infrequently; sacrococcygeal teratoma (SCT) is a rare and abnormal mass vided in this issue. The often diagnosed on antenatal ultrasound. An SCT may cause serious antenatal complications, requires posttest and evaluation must be com- surgery in the neonatal period, and can lead to various long-term sequelae including fecal incontinence pleted online. Details to complete the course are provided online at acade- or constipation, urinary incontinence, and lower extremity mobility impairment. Even rarer are SCTs myonline.org. Sign into your ANN that include intraspinal extension necessitating complex neurosurgical intervention to relieve possible account or register for a non-member spinal cord compression or tumor tissue resection. A comprehensive understanding of the natural account if you are not a member. A total of 1.5 contact hour(s) can be earned as history of SCT provides frontline neonatal nurses and nurse practitioners with the expertise and CNE credit for reading this article, language to support families during an infant’s NICU admission. A glossary of key terms accompanied studying the content, and completing the online posttest and evaluation. To by a case review of a premature infant born with a large external SCT with intrapelvic and intraspinal be successful, the learner must obtain a components aids in enhancing knowledge related to the potential impact of an SCT on the central grade of at least 80 percent on the test. -

Presacral Retroperitoneal Approach to Axial Lumbar Interbody Fusion: A
Presacral retroperitoneal approach to axial lumbar interbody fusion: a new, minimally invasive technique at L5-S1: Clinical outcomes, complications, and fusion rates in 50 patients at 1-year follow-up Robert J. Bohinski, Viral V. Jain and William D. Tobler Int J Spine Surg 2010, 4 (2) 54-62 doi: https://doi.org/10.1016/j.esas.2010.03.003 http://ijssurgery.com/content/4/2/54 This information is current as of September 25, 2021. Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://ijssurgery.com/alerts The International Journal of Spine Surgery 2397 Waterbury Circle, Suite 1, Aurora, IL 60504, Phone: +1-630-375-1432 © 2010 ISASS. All Rights Reserved. Downloaded from http://ijssurgery.com/ by guest on September 25, 2021 Available online at www.sciencedirect.com SAS Journal 4 (2010) 54–62 www.sasjournal.com Presacral retroperitoneal approach to axial lumbar interbody fusion: a new, minimally invasive technique at L5-S1: Clinical outcomes, complications, and fusion rates in 50 patients at 1-year follow-up Robert J. Bohinski, MD, PhD a, Viral V. Jain, MD b, William D. Tobler, MD a,* a Department of Neurosurgery, University of Cincinnati (UC) Neuroscience Institute, UC College of Medicine, Mayfield Clinic and Spine Institute, and The Christ Hospital, Cincinnati, OH b Department of Orthopedic Surgery, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH Abstract Background: The presacral retroperitoneal approach to an axial lumbar interbody fusion (ALIF) is a percutaneous, minimally invasive technique for interbody fusion at L5-S1 that has not been extensively studied, particularly with respect to long-term outcomes. -

Neurological Manifestation of Sacral Tumors
Neurosurg Focus 15 (2):Article 1, 2003, Click here to return to Table of Contents Neurological manifestation of sacral tumors MICHAEL PAYER, M.D. Department of Neurosurgery, University Hopital of Geneva, Switzerland An extensive analysis of the existing literature concerning sacral tumors was conducted to characterize their clin- ical manifestations. Although certain specific manifestations can be attributed to some of the tumor types, a more general pattern of clinical presentation of an expansive sacral lesion can be elaborated. Local pain with or without pseudoradicular or radicular radiation is the most frequent initial symptom and is usually followed by the manifesta- tion of a lumbosacral sensorimotor deficit; bladder/bowel and/or sexual dysfunction appear throughout the natural course of disease. KEY WORDS • sacrum • tumor • lesion • neurological presentation All sacral and presacral tumors are rare.32,93 In one se- REVIEW OF SACRAL ANATOMY ries patients with these tumors were estimated to account for approximately one in 40,000 hospital admissions.93 Osseous Structures of the Sacrum Tumors arising from the bone of the sacrum are by far the The sacrum is a complex bone, comprising five sacral most frequent sacral tumors; chordomas are the most com- vertebrae that have fused. In its center lies the longitudi- mon and GCTs the second most common.20,46,50,61,74,81,98 nal sacral canal, which opens caudally posteriorly into the Although sacrococcygeal teratoma is the most common sacral hiatus, an incomplete posterior closure of the S-5 sacral tumor in neonates, it is very rare in adults.30,45,66 lamina. The thick anterior or pelvic face of the sacrum is The author conducted an extensive analysis of the exist- concave and contains four right- and left-sided anterior ing literature concerning tumors of the sacrum to charac- sacral foramina. -

Reoperative Pelvic Surgery
gastrointestinal tract and abdomen REOPERATIVE PELVIC SURGERY Eric J. Dozois, MD, FACS, FASCRS, and Daniel I. Chu, MD Reoperative pelvic surgery is technically challenging and nerve roots, sciatic nerve, piriformis, obturator internus carries with it signifi cant potential risk. The inherent risks of muscles, and ligaments, including the sacrotuberous and any pelvic operation, such as bleeding and injury to critical sacrospinous. For extended sacropelvic resections, an expe- structures such as the ureters, are magnifi ed by the oblitera- rienced orthopedic oncologic spine surgeon greatly enhance s tion of previous embryonic fusion planes and anatomic the ability to preserve important nerve roots and ensure relationships within the confi nes of a narrow, deep opera- adequate oncologic musculoskeletal margins. tive fi eld. This topic review addresses the surgical indica- tions, techniques, and pitfalls when dealing with recurrent Operative Management pelvic pathology and provides an overview of safe and effective approaches to reoperative pelvic surgery. recurrent malignancy: rectal cancer Pelvic pathology that requires reoperative surgery in the gastrointestinal tract usually involves four surgical themes: Despite modern management, locally recurrent rectal (1) recurrent malignancies, for which we use recurrent rectal cancer remains a signifi cant problem, with the incidence of 1–3 cancer as an example; (2) complications from ileal pouch- recurrence as high as 33% in some series. Only approxi- anal anastomoses (IPAAs) for infl ammatory bowel disease; mately 20% of patients with recurrent disease, however, 4 (3) complications from low pelvic anastomoses; and (4) pal- may be amenable to repeat curative resection. Although the liative situations. For any of these indications, the goals of incidence of metastatic disease in recurrent rectal cancer reoperative pelvic surgery are twofold: (1) resection/repair approaches 70%, up to 50% of patients die with local disease 2,5–9 of the primary indication, which could include pelvic exen- only. -
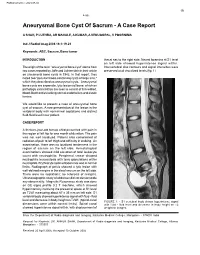
Aneurysmal Bone Cyst of Sacrum - a Case Report
Published online: 2021-08-02 19 4-66 Aneurysmal Bone Cyst Of Sacrum - A Case Report A SHAH, P LUTHRA, AR MAHALE, A KUMAR, A VENUGOPAL, V POORNIMA Ind J Radiol Imag 2006 16:1:19-21 Keywords: ABC, Sacrum, Bone tumor INTRODUCTION thecal sac to the right side. Neural foramina at S1 level on left side showed hyperintense signal within. The origin of the term "aneurysmal bone cyst" stems from Intervertebral disc contours and signal intensities were two cases reported by Jaffe and Lichtenstein in their article preserved at all visualized levels (fig. 1). on unicameral bone cysts in 1942. In that report, they noted two "peculiar blood-containing cysts of large size," which they described as aneurysmal cysts. Aneurysmal bone cysts are expansile, lytic lesions of bone, which on pathologic examination are seen to consist of thin-walled, blood-filled cavities lacking normal endothelium and elastic lamina. We would like to present a case of aneurysmal bone cyst of sacrum. A rare presentation of the lesion in the vertebral body with no/minimal septations and distinct fluid-fluid level in our patient. CASE REPORT A thirteen-year-old female child presented with pain in the region of left hip for one month of duration. The pain was not well localized. Patient also complained of radiation of pain to left thigh and difficulty in walking. On examination, there was no localized tenderness in the region of sacrum on the left side. Hematological examinations showed mild elevation of total leukocyte count with neutrophilia. Peripheral smear showed neutrophilic leucocytosis with toxic granulations within neutrophils. -
A Case Report Involving the Spine and Soft Tissues
University of Kentucky UKnowledge Radiology Faculty Publications Radiology 6-14-2021 Extrapulmonary Tuberculosis: A Case Report Involving the Spine and Soft Tissues Aden Gunnar Miller University of Kentucky, [email protected] Paul J. Spicer University of Kentucky, [email protected] Follow this and additional works at: https://uknowledge.uky.edu/radiology_facpub Part of the Radiology Commons Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Repository Citation Miller, Aden Gunnar and Spicer, Paul J., "Extrapulmonary Tuberculosis: A Case Report Involving the Spine and Soft Tissues" (2021). Radiology Faculty Publications. 34. https://uknowledge.uky.edu/radiology_facpub/34 This Article is brought to you for free and open access by the Radiology at UKnowledge. It has been accepted for inclusion in Radiology Faculty Publications by an authorized administrator of UKnowledge. For more information, please contact [email protected]. Extrapulmonary Tuberculosis: A Case Report Involving the Spine and Soft Tissues Digital Object Identifier (DOI) https://doi.org/10.1016/j.radcr.2021.05.049 Notes/Citation Information Published in Radiology Case Reports, v. 16, issue 8. © 2021 The Authors This is an open access article under the CC BY-NC-ND license (https://creativecommons.org/licenses/by- nc-nd/4.0/). This article is available at UKnowledge: https://uknowledge.uky.edu/radiology_facpub/34 Radiology Case Reports 16 (2021) 2236–2239 Available online at www.sciencedirect.com journal homepage: www.elsevier.com/locate/radcr Case report Extrapulmonary tuberculosis: a case report ✩ involving the spine and soft tissues ∗ Aden Gunnar Miller, BS a, , Paul J. -
The Anatomical Compartments and Their Connections As Demonstrated by Ectopic Air
Insights Imaging (2013) 4:759–772 DOI 10.1007/s13244-013-0278-0 PICTORIAL REVIEW The anatomical compartments and their connections as demonstrated by ectopic air Ana Frias Vilaça & Alcinda M. Reis & Isabel M. Vidal Received: 4 April 2013 /Revised: 10 July 2013 /Accepted: 17 July 2013 /Published online: 25 September 2013 # The Author(s) 2013. This article is published with open access at Springerlink.com Abstract Air/gas outside the aero-digestive tract is abnor- Keywords Subcutaneous emphysema . Pneumomediastinum mal; depending on its location, it is usually called emphy- Pneumoretroperitoneum . Fascia . Anatomy sema, referring to trapped air/gas in tissues, or ectopic air/ gas. It can be associated to a wide range of disorders, and although it usually is an innocuous condition, it should Introduction prompt a search for the underlying aetiology, since some of its causes impose an urgent treatment. In rare instances, Air/gas is normally seen in some body structures, such as it may itself represent a life-threatening condition, in paranasal sinuses, and in the respiratory and gastroin- depending on the site involved and how quickly it testinal tract. However, when present in subcutaneous tis- evolves. Abnormal air/gas beyond viscera and serosal sues, cervical, mediastinal, retroperitoneal, extraperitoneal spaces, reaches its location following some anatomic abdomen and pelvis spaces, involving muscular fibres or boundaries, such as fascia, which may help search the interstitial tissues, it is abnormal, indicating a “pathological source; however if the air pressure exceeds the strength process”, and represents a challenge to search for the of the tissues, or the time between the aggression and the underlying aetiology. -

(12) United States Patent (10) Patent No.: US 7,641,657 B2 Cragg (45) Date of Patent: *Jan.5, 2010
USOO7641657B2 (12) United States Patent (10) Patent No.: US 7,641,657 B2 Cragg (45) Date of Patent: *Jan.5, 2010 (54) METHOD AND APPARATUS FOR 3,367,326 A 2, 1968 Frazier ........................ 606/61 PROVIDING POSTERIOR OR ANTERIOR TRANS-SACRAL ACCESS TO SPINAL VERTEBRAE (Continued) (75) Inventor: Andrew H. Cragg, Edina, MN (US) FOREIGN PATENT DOCUMENTS - DE 91 08 043 10, 1991 (73) Assignee: TranS1, Inc., Wilmington, NC (US) (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 (Continued) This patent is Subject to a terminal dis- Michael MacMilland, MD, et al., “Percutaneous Lumbosacral Fixa claimer. tion and Fusion.” Percutaneous Spine Techniques, Jan. 1996, vol. 7. No. 1, pp. 99-106. (21) Appl. No.: 11/462.934 (Continued) (22) Filed: Aug. 7, 2006 Primary Examiner Pedro Philogene (74) Attorney, Agent, or Firm Knobbe Martens Olson & (65) Prior Publication Data Bear LLP US 2007/OO55260 A1 Mar. 8, 2007 (57) ABSTRACT Related U.S. Application Data (63) Continuation of application No. 10/459,149, filed on Methods and apparatus for providing percutaneous access to Jun. 10, 2003, now Pat. No. 7,087.058. Vertebrae in alignment with a visualized, trans-sacral axial s s sw Y- s instrumentation/fusion (TASIF) line in a minimally invasive, (51) Int. Cl. low trauma, manner are disclosed. A number of related TASIF A6B I7/00 (2006.01) methods and Surgical tool sets are provided by the present invention that are employed to form a percutaneous pathway (52) U.S. Cl. ....................................... 606/79;606/86 R from an anterior or posterior skin incision to a respective (58) Field of Classification Search .................. -

Currarino Syndrome: Repair of the Dysraphic Anomalies and Resection of the Presacral Mass in a Combined Neurosurgical and General Surgical Approach
CLINICAL ARTICLE J Neurosurg Pediatr 22:584–590, 2018 Currarino syndrome: repair of the dysraphic anomalies and resection of the presacral mass in a combined neurosurgical and general surgical approach Michael D. Cearns, MBBS, BSc,1 Samantha Hettige, FRCS(SN),1 Paolo De Coppi, MD, PhD,2 and Dominic N. P. Thompson, FRCS(SN)1 1Department of Paediatric Neurosurgery and 2Specialist Neonatal and Paediatric Surgery, Great Ormond Street Hospital, London, United Kingdom OBJECTIVE It is well established that Currarino syndrome (CS) may be associated with spinal dysraphism. Here, the authors report on 10 CS patients with dysraphic anomalies who had undergone a combined neurosurgical and general surgical approach to repair the dysraphic anomalies and resect the presacral mass in a single operation. They discuss the spectrum of spinal dysraphism that may coexist in CS in the context of its developmental etiology. METHODS Children with a confirmed CS diagnosis who had undergone the combined operative approach were identi- fied from a departmental database. Presenting features were recorded and preoperative imaging was analyzed to record features of the presacral mass and the dysraphic anomalies. The histopathological nature of the resected presacral mass and the outcomes postoperatively and at the last follow-up were reviewed. RESULTS Between 2008 and 2015, 10 patients presented with CS, 9 with constipation. Median age at the time of surgery was 1.3 years. Six of the 10 patients had anorectal malformation consisting of anal stenosis, rectal stenosis, or imperforate anus. Spinal anomalies included anterior meningocele (5 cases), low-lying conus (8), terminal syrinx (4), fatty filum (5), caudal lipoma (3), and intraspinal cyst (1). -
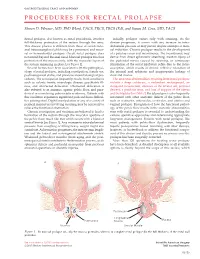
Procedures for Rectal Prolapse
gastrointestinal tract and abdomen PROCEDURES FOR RECTAL PROLAPSE Steven D. Wexner, MD, PhD (Hon), FACS, FRCS, FRCS (Ed), and Susan M. Cera, MD, FACS Rectal prolapse, also known as rectal procidentia, involves Initially, prolapse occurs only with straining. As the full-thickness protrusion of the rectum through the anus. disease progresses, it occurs with any increase in intra- This disease process is different from those of occult recto- abdominal pressure or may persist despite attempts at man- anal intussusception (which may be a precursor) and muco- ual reduction. Chronic prolapse results in the development sal or hemorrhoidal prolapse. Occult rectal prolapse does of a patulous anus and incontinence. The incontinence may not extend beyond the anal canal. Mucosal prolapse involves derive from direct sphincteric stretching, traction injury of protrusion of the mucosa only, with the muscular layers of the pudendal nerves caused by straining, or continuous the rectum remaining in place [see Figure 1]. stimulation of the rectal inhibitory reflex due to the intus- Several factors have been associated with the pathophysi- susception, which results in chronic reflexive relaxation of ology of rectal prolapse, including constipation, female sex, the internal anal sphincter and inappropriate leakage of postmenopausal status, and previous anorectal surgical pro- stool and mucus. cedures. The constipation frequently results from conditions The anatomic abnormalities resulting from rectal prolapse such as colonic inertia, neurologic disease, psychiatric ill- include a deep cul-de-sac, a redundant rectosigmoid, an ness, and obstructed defecation. Obstructed defecation is elongated mesorectum, diastasis of the levator ani, perineal also referred to as anismus, spastic pelvic floor, and para- descent, a patulous anus, and loss of support of the uterus doxical or nonrelaxing puborectalis syndrome. -

Neuroendocrine Tumour Developing Within a Long-Standing Tailgut Cyst
Clinical Journal of Gastroenterology (2019) 12:539–551 https://doi.org/10.1007/s12328-019-00998-4 CASE REPORT Neuroendocrine tumour developing within a long‑standing tailgut cyst: case report and review of the literature Alice Lee1 · Thomas Surya Suhardja1,2 · Thang Chien Nguyen1,2 · William Meng‑Keat Teoh1,2 Received: 22 February 2019 / Accepted: 22 May 2019 / Published online: 30 May 2019 © Japanese Society of Gastroenterology 2019 Abstract A tailgut cyst is a rare congenital lesion that can develop in the presacral space from the remnants of an embryonic hindgut. It is unusual for malignant change to occur in a tailgut cyst. We report a case of a large long-standing tailgut cyst, which was removed during a laparotomy. Histopathology showed a well-diferentiated neuroendocrine tumour (primary carcinoid tumour) arising in a tailgut cyst. We reviewed the English literature for all adult cases with this condition. All original articles were reviewed, and data were compiled and tabulated. Including this report, 29 cases of NET developing in a tailgut cyst were found in the English literature. Tailgut cysts have been reported as more common in females, with a mean age of presentation in the ffth decade (Devine, in: Zbar A, Wexner S (eds) Coloproctology. Springer specialist surgery series, Springer, London, 2010; Hjermstad and Helwig in Am J Clin Pathol 89:139–147, 1988). Tailgut cysts may undergo malignant change including adenocarcinoma, sarcoma, and NET (Mathis et al. Br J Surg 97:575–579, 2010; Messick in Dis Colon Rectum 61:151–153, 2018; Patsouras et al. in Colorectal Dis 17:724–729, 2015; Chereau et al in Colorectal Dis 15:e476–e482, 2013). -

Presacral Anterior Lumbar Interbody Fusion
145 PRESACRAL ANTERIOR Aspects Spine Surgery: Current Minimally Invasive LUMBAR INTERBODY FUSION (AXIALIF), MINI ALIF 25 Bayram Cırak MD ack pain is one of the most common condi- autograft (patient’s own bone source), or allograft tions in any population and have a variety from a donor.7-10 1 of etiologies. The costs for treatment and The first lumbar anterior interbody fusion was B 2 absence from work are tremendous. Low back pain reported in the 1930s.11 That was open technique. originates from paraspinal muscles, facet joints, spi- The development of new techniques and technol- nal ligaments, failed endplates, or degenerative in- ogies and better understanding of surgical anat- tervertebral discs.3 Current strategy for back pain omy gave rise to minimally invasive spine surgery aims to alleviate the symptom and to resolve eti- (MISS).11-13 ology itself. Surgical options have been considered 4 when conservative treatment fails. Conservative A. Presacral ALIF (anterior lumbar interbody therapies include reduced activity and bed rest, analgesics, muscle relaxants, nonsteroidal antiin- fusion) flammatory drugs (NSAID) and/or rehabilitation Presacral ALIF or AXIALIF is performed for the in- programs.5 When the conservative therapies fail, terbody fusion of the lower lumbar vertebrae. In- Patients undergo a surgical treatment, which in- stability due to degenerative disc disease and spon- ludes decompression, stabilisation and / or fusion.6 dylolisthesis is frequently seen at L4–L5 and L5–S1 Spinal fusion is the surgical connection of two or levels. which may require fusion to achieve stability more adjacent vertebrae to immobilise. To relieve and relieve symptoms.