Evaluation of Smartstax and Smartstax PRO Maize Against Western Corn Rootworm and Northern Corn Rootworm: Efficacy and Resistance Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
U.S. V. Bayer AG and Monsanto Company Comment: the Sierra Club
ATTN: Kathleen S. O'Neill Chief, Transportation, Energy & Agriculture Section Antitrust Division United States Department of Justice 450 5th Street, NW, Suite 800 Washington, DC 20530 Petition in opposition to proposed U.S. v. Bayer AG and Monsanto Company settlement and merger: A merger of agrochemical giants Bayer and Monsanto would create the world's largest seed and pesticide maker. I am afraid this move will reduce competition, raise prices for consumers and farmers, and result in an unacceptable degree of control over the agricultural industry and our food supply. I am very concerned about pollinators and the increased risks to bees, butterflies and birds with the increase of Bayer's neonicotinoids. Both companies produce corn products engineered to imply the use of harmful pesticides they manufacture. The production of corn uses high amounts of nitrogen- based fertilizers and the excess sediment is contaminating our waterways, therefore I am deeply worried about increased corn production from this merger. The heavy nutrient runoff from corn is widely attributed to exacerbating the marine "Dead Zone" in the Gulf of Mexico, in which algal blooms create hypoxic conditions wherein oxygen concentration is in such low levels that marine life suffocates and dies. I urge the Department of Justice to do more prevent the Bayer-Monsanto seed and pesticide platform from growing too strong by stopping this merger. If this merger is allowed, it should require more pesticide and seed divestments in order to protect our agriculture and food supply. This merger is anti-competition, if it is approved it will fail to protect farmers, consumers and the environment by allowing further consolidation of the industrial agriculture sector. -

Plant Molecular Farming: a Viable Platform for Recombinant Biopharmaceutical Production
plants Review Plant Molecular Farming: A Viable Platform for Recombinant Biopharmaceutical Production Balamurugan Shanmugaraj 1,2, Christine Joy I. Bulaon 2 and Waranyoo Phoolcharoen 1,2,* 1 Research Unit for Plant-Produced Pharmaceuticals, Chulalongkorn University, Bangkok 10330, Thailand; [email protected] 2 Department of Pharmacognosy and Pharmaceutical Botany, Faculty of Pharmaceutical Sciences Chulalongkorn University, Bangkok 10330, Thailand; [email protected] * Correspondence: [email protected]; Tel.: +66-2-218-8359; Fax: +66-2-218-8357 Received: 1 May 2020; Accepted: 30 June 2020; Published: 4 July 2020 Abstract: The demand for recombinant proteins in terms of quality, quantity, and diversity is increasing steadily, which is attracting global attention for the development of new recombinant protein production technologies and the engineering of conventional established expression systems based on bacteria or mammalian cell cultures. Since the advancements of plant genetic engineering in the 1980s, plants have been used for the production of economically valuable, biologically active non-native proteins or biopharmaceuticals, the concept termed as plant molecular farming (PMF). PMF is considered as a cost-effective technology that has grown and advanced tremendously over the past two decades. The development and improvement of the transient expression system has significantly reduced the protein production timeline and greatly improved the protein yield in plants. The major factors that drive the plant-based platform towards potential competitors for the conventional expression system are cost-effectiveness, scalability, flexibility, versatility, and robustness of the system. Many biopharmaceuticals including recombinant vaccine antigens, monoclonal antibodies, and other commercially viable proteins are produced in plants, some of which are in the pre-clinical and clinical pipeline. -

Genuity Smartstax Corn: an Amazing Advancement in GE Crop
Genuity SmartStax corn: An amazing advancement in GE crop 05 May 2010 | News Image not found or type unknown Using GS corn as a platform would be a quicker way of enhancing the number of transgenes in a single crop variety The trademarked Genuity-SmartStax corn (GS corn) containing eight transgenes-six for pest control and two for weed control- developed through collaboration between Monsanto and Dow AgroSciences, introduced few months ago, is an amazing development in crop genetic engineering (GE). Incorporated into the best of corn varieties, this event is expected to provide the most comprehensive pest and weed control system available, leading to an impressive crop health and increase of whole farm crop yields. The development of Genuity SmartStax corn from the shaky origins of genetic engineering is a fascinating reading. Genuity SmartStax corn GS corn takes care of the major pests, such as the European and southwestern corn borer, northern and western corn rootworm, western bean cutworm, black cutworm, corn earworm, and fall armyworm and also imparts tolerance to both glyphosate and glufosinate herbicides. In addition, the coming together of two giants in the seed industry will encourage other private-private partnerships to further this initiative. Eight transgenes in GS corn Tolerance to aerial pests (three Bt genes): Cry 1A.105 (Monsanto), Cry 2Ab2 (Monsanto) and Cry 1F (Dow). Tolerance to subsoil pests (three Bt genes): Cry 3Bb1 (Monsanto), Cry 34Ab1 (Dow) and Cry 35Ab1 (Dow). Tolerance to herbicides (two genes): Glyphosate (Roundup Ready, Monsanto) and Glufosinate (LibertyLink, Dow, under license from Bayer). Biosecurity evaluation Transgenic crops are evaluated for product efficacy and biosecurity in the laboratory, green house and in the field for over 10 years before commercialization. -

US EPA, Pesticide Product Label, WIDESTRIKE 3 INSECT
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION October 18, 2016 Stephanie L. Burton US Regulatory Manager Dow AgroSciences LLC 9330 Zionsville Road Indianapolis, IN 46268-1054 Subject: PRIA (Pesticide Registration Improvement Act) Amendment – to update the terms of registration related to gene flow and revise the product label. Product Name: WideStrike® 3 Insect Resistant Cotton EPA Registration Number: 68467-19 Application Date: June 23, 2016 OPP Decision Number: 518794 Dear Ms. Burton: The amendment referred to above, submitted in connection with registration under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), as amended, are acceptable provided you comply with the revised terms of registration as set forth below: 1. Submit/cite all data required for registration of your product under FIFRA § 3(c)(5) when the Agency requires all registrants of similar products to submit such data. 2. Gene Flow The following information regarding commercial production must be included in the grower guide for WideStrike® 3 Insect Resistant Cotton: a) No planting of WideStrike® 3 Insect Resistant Cotton is permitted south of Route 60 (near Tampa) in Florida. b) Commercial culture of WideStrike® 3 Insect Resistant Cotton is prohibited in Hawaii, Puerto Rico, and the US Virgin Islands. The following information regarding test plots and seed production must appear in contracts or on bags of WideStrike® 3 Insect Resistant Cotton intended for the following purposes: Page 2 of 10 EPA Reg. No. 68467-19 OPP Decision No. 518794 a) Test plots or breeding nurseries, regardless of the plot size, established in Hawaii must not be planted within 3 miles of Gossypium tomentosum. -

Monsanto Company in Microsoft Word Format Together with a Copy of the Transmittal Letter That Accompanies the Filing of Two Paper Copies of the Submission
From: Letzler, Kenneth [mailto:[email protected]] Sent: Thursday, December 31, 2009 1:03 PM To: ATR-Agricultural Workshops Subject: Comment Attached please find a comment submitted on behalf of Monsanto Company in Microsoft Word format together with a copy of the transmittal letter that accompanies the filing of two paper copies of the submission. _____________________________ U.S. Treasury Circular 230 Notice Any U.S. federal tax advice included in this communication (including any attachments) was not intended or written to be used, and cannot be used, for the purpose of (i) avoiding U.S. federal tax-related penalties or (ii) promoting, marketing or recommending to another party any tax-related matter addressed herein. _____________________________ This communication may contain information that is legally privileged, confidential or exempt from disclosure. If you are not the intended recipient, please note that any dissemination, distribution, or copying of this communication is strictly prohibited. Anyone who receives this message in error should notify the sender immediately by telephone or by return e-mail and delete it from his or her computer. ---------------------------------------------------------------------- For more information about Arnold & Porter LLP, click here: http://www.arnoldporter.com Competition and Innovation in American Agriculture A Response to the American Antitrust Institute’s “Transgenic Seed Platforms: Competition Between a Rock and a Hard Place?” Submitted on Behalf of Monsanto Company In Response to the Request for Comments by the United States Department of Agriculture and United States Department of Justice, Antitrust Division, in Connection with Their Hearings on “Agriculture and Antitrust Enforcement Issues in Our 21st Century Economy” Vandy Howell, Ph.D. -

Dow Agrosciences
Dow AgroSciences LLC (Dow AgroSciences) markets crop protection Dow AgroSciences products and seeds for a broad spectrum of crops, including maize, soybean, cotton and forage. The company began in the 1950s as the agricultural unit of The Dow Chemical Company. As a joint venture Corporate Data of The Dow Chemical Company and Eli Lilly & Co., it was known as Headquarters: Indianapolis, Indiana, USA DowElanco from 1989 onwards. In 1997, The Dow Chemical Company Ownership type: Listed Group revenue (2014): USD 729,000,0000 acquired 100% ownership.* Global Index – Commitment Performance Transparency Innovation Field Crop Seed Companies 1.27 1.46 2.05 0.25 Dow AgroSciences ranks in the lower 5 range of the Global Index of Field Crop Seed Companies. It has clear rank out of 7 approaches to Public Policy & Stakeholder A Governance & 1.13 score 1.38 Engagement and existing breeding Strategy B Public Policy & 2.68 programs for resistance to pests and Stakeholder Engagement diseases, abiotic stress tolerance and C Genetic Resources & 1.09 Intellectual Property yield, although it is not clear to what extent these programs D Research & 1.31 specifically target the development of varieties suitable for Development E Marketing & 1.11 Index countries and smallholder farmers. Seed sales were Sales found only in Latin American Index countries. Given the F Capacity 2.08 indications of research and capacity-building activities rel- Building G Local Seed Sector 0.91 evant for improved access to seeds for smallholder farmers in Advancement other regions, the company is encouraged to develop its seed 0 1 2 3 4 5 business serving smallholder farmers on a more global scale. -

The Era of Corporate Consolidation and the End of Competition Bayer-Monsanto, Dow-Dupont, and Chemchina-Syngenta
Research Brief October 2018 The Era of Corporate Consolidation and the End of Competition Bayer-Monsanto, Dow-DuPont, and ChemChina-Syngenta DISRUPT ECOSYSTEM ACCLERATE MONOPOLY THE EFFECTS OF CORPORATE CONSOLIDATION UNDERMINE FOOD SECURITY HARM SMALL PRODUCERS HAASINSTITUTE.BERKELEY.EDU This publication is published by the Haas Institute for a Fair and Inclusive Society at UC Berkeley This research brief is part of the Haas Institute's Shahidi Project from the Global Justice Program. The Shahidi Project (Shahidi is a Swahili word meaning “witness”) intends to demystify the power structures and capacities of transnational food and agricultural corporations within our food system. To that end, researchers have developed a robust database focusing on ten of the largest food and agricultural corporations in the world. See more at haasinstitute.berkeley.edu/shahidi. About the Authors Copyeditor Support Elsadig Elsheikh is the director Marc Abizeid Special thanks to the Food of the Global Justice program and Farm Communications at the Haas Institute for a Infographics Fund, which provided the seed Fair and Inclusive Society at Samir Gambhir funding for the Shahidi project. the University of California- Berkeley, where he oversees Report Citation Contact the program’s projects and Elsadig Elsheikh and Hossein 460 Stephens Hall research on corporate power, Ayazi. “The Era of Corporate Berkeley, CA 94720-2330 food system, forced migration, Consolidation and The End of Tel 510-642-3326 human rights, Islamophobia, Competition: Bayer-Monsanto, haasinstitute.berkeley.edu structural marginality and Dow-DuPont, and ChemChina- inclusion, and trade and Syngenta.” Haas Institute for development. a Fair and Inclusive Society at the University of California, Hossein Ayazi, PhD, is a Berkeley, CA. -

Dow Agrosciences Petitions (09-233- 01P, 09-349-01P, and 11-234-01P) for Determinations of Nonregulated Status for 2,4-D-Resistant Corn and Soybean Varieties
Dow AgroSciences Petitions (09-233- 01p, 09-349-01p, and 11-234-01p) for Determinations of Nonregulated Status for 2,4-D-Resistant Corn and Soybean Varieties Draft Environmental Impact Statement—2013 Agency Contact: Sid Abel Biotechnology Regulatory Services 4700 River Road USDA, APHIS Riverdale, MD 20737 Fax: (301) 734-6352 The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, or marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’S TARGET Center at (202) 720–2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326–W, Whitten Building, 1400 Independence Avenue, SW, Washington, DC 20250–9410 or call (202) 720–5964 (voice and TDD). USDA is an equal opportunity provider and employer. Mention of companies or commercial products in this report does not imply recommendation or endorsement by the U.S. Department of Agriculture over others not mentioned. USDA neither guarantees nor warrants the standard of any product mentioned. Product names are mentioned solely to report factually on available data and to provide specific information. This publication reports research involving pesticides. All uses of pesticides must be registered by appropriate State and/or Federal agencies before they can be recommended. CAUTION: Pesticides can be injurious to humans, domestic animals, desirable plants, and fish and other wildlife—if they are not handled or applied properly. -
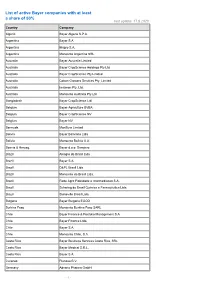
List of Active Bayer Companies with at Least a Share of 50% Last Update: 17.8.2020 Country Company
List of active Bayer companies with at least a share of 50% last update: 17.8.2020 Country Company Algeria Bayer Algerie S.P.A. Argentina Bayer S.A. Argentina Biagro S.A. Argentina Monsanto Argentina SRL Australia Bayer Australia Limited Australia Bayer CropScience Holdings Pty Ltd Australia Bayer CropScience Pty Limited Australia Cotton Growers Services Pty. Limited Australia Imaxeon Pty. Ltd. Australia Monsanto Australia Pty Ltd Bangladesh Bayer CropScience Ltd. Belgium Bayer Agriculture BVBA Belgium Bayer CropScience NV Belgium Bayer NV Bermuda MonSure Limited Bolivia Bayer Boliviana Ltda Bolivia Monsanto Bolivia S.A. Bosnia & Herzeg. Bayer d.o.o. Sarajevo Brazil Alkagro do Brasil Ltda Brazil Bayer S.A. Brazil D&PL Brasil Ltda Brazil Monsanto do Brasil Ltda. Brazil Rede Agro Fidelidade e Intermediacao S.A. Brazil Schering do Brasil Química e Farmacêutica Ltda. Brazil Stoneville Brasil Ltda. Bulgaria Bayer Bulgaria EOOD Burkina Faso Monsanto Burkina Faso SARL Chile Bayer Finance & Portfolio Management S.A. Chile Bayer Finance Ltda. Chile Bayer S.A. Chile Monsanto Chile, S.A. Costa Rica Bayer Business Services Costa Rica, SRL Costa Rica Bayer Medical S.R.L. Costa Rica Bayer S.A. Curacao Pianosa B.V. Germany Adverio Pharma GmbH - 1 - List of active Bayer companies with at least a share of 50% last update: 17.8.2020 Country Company Germany AgrEvo Verwaltungsgesellschaft mbH Germany Alcafleu Management GmbH & Co. KG Germany BGI Deutschland GmbH Germany Bayer 04 Immobilien GmbH Germany Bayer 04 Leverkusen Fußball GmbH Germany Bayer 04 Leverkusen -

Whither Plant Genetic Engineering? Allow Crops to Tolerate Environmental Stress Such As Drought, Cold, Salt, Heat, Or flood
PLANT TREK TO BOLDLY GO WHERE NO PLANT HAS GONE BEFORE On the Past, Present & Future of Plant Genetic Engineering by Richard G. Stout A HowPlantsWork.com eBook Copyright © 2013 by Richard G. Stout Version 1.0.1 PDF August, 2013 Table of Contents Preface Chapter 1: Where Do New Plants Come From? Chapter 2: How To Make A Transgenic Plant Chapter 3: Gene Guns, Terminators & Traitors Chapter 4: Farmaceuticals, Plantibodies & Edible Vaccines Chapter 5: Into The Wild Chapter 6: Are GM Plants Self-Replicating Inventions? Chapter 7: Plant Trek - The Next Generation Chapter 8: DIY Plant Genetic Engineering? Attributions About The Author Glossary about where plant biotechnology may be headed in the future, Preface including how plant biotechnology “hobbyists” may be getting into the act. Who is this book for? Please Note: This book is NOT a comprehensive textbook on plant genetic engineering and biotechnology. (If you’re looking This book is intended for people who may be curious about for such books, I’m sure you can find them at your local college plant genetic engineering, but who don’t want to read a long, bookstore or at an online bookseller.) Nor is this book meant to technical textbook on the subject. (There are provided, be a defense of genetically-engineered organisms (GMOs), however, ample links to books and articles - and also to online though I’m sure some readers will think so. Maybe here’s why. resources - for further reading.) If you’re looking for small “tastes” of information regarding various aspects of plant Since I was a graduate student in the 1970s at the University of genetic engineering, then this little book maybe just the Washington where some of the original work on transgenic informational “snack” that you’re looking for. -

Agribusiness and Antitrust: the Bayer-Monsanto Merger, Its Legality, and Its Effect on the United States and European Union
The Global Business Law Review Volume 7 Issue 1 Article 9 7-1-2018 Agribusiness and Antitrust: The Bayer-Monsanto Merger, Its Legality, and Its Effect on the United States and European Union Aleah Douglas Cleveland-Marshall College of Law Follow this and additional works at: https://engagedscholarship.csuohio.edu/gblr Part of the Antitrust and Trade Regulation Commons, and the Business Organizations Law Commons How does access to this work benefit ou?y Let us know! Recommended Citation Aleah Douglas, Agribusiness and Antitrust: The Bayer-Monsanto Merger, Its Legality, and Its Effect on the United States and European Union, 7 Global Bus. L. Rev. 156 (2018) available at https://engagedscholarship.csuohio.edu/gblr/vol7/iss1/9 This Note is brought to you for free and open access by the Journals at EngagedScholarship@CSU. It has been accepted for inclusion in The Global Business Law Review by an authorized editor of EngagedScholarship@CSU. For more information, please contact [email protected]. AGRIBUSINESS AND ANTITRUST: THE BAYER-MONSANTO MERGER, ITS LEGALITY, AND ITS EFFECT ON THE UNITED STATES AND EUROPEAN UNION ALEAH DOUGLAS I. INTRODUCTION……………………………………………………………………... 157 II. BACKGROUND…………………………………………………………………….....158 A. A Review of the Bayer-Monsanto Merger………………………….…………... 158 B. United States Antitrust Laws…………………………………………………… 161 1. The Applicable Laws……………………………………………………. 161 2. Problems and Antitrust Violations……………………………………… 166 3. American Agribusiness…………………………………………………. 167 C. European Union Antitrust Laws……………………………………………….. 172 1. The European Union……………………………………………………. 172 2. The Applicable Laws………………………………….………………… 172 3. Problems and Antitrust Violations……………………….………....…... 174 4. European Union Agribusiness…………….…………………………….. 176 D. Illegality and Detriment …………………………………….…………………. 178 III. CONCLUSION……………………………………………………………………....... 180 ABSTRACT This note examines the current and historical antitrust laws of the United States and the European Union as they relate to the currently pending merger between Bayer and Monsanto. -

Department of Entomology Newsletter for Alumni and Friends (2011) Iowa State University, Department of Entomology
Department of Entomology Newsletter Entomology 1-2011 Department of Entomology Newsletter For Alumni and Friends (2011) Iowa State University, Department of Entomology Follow this and additional works at: http://lib.dr.iastate.edu/entnewsletter Part of the Entomology Commons Recommended Citation Iowa State University, Department of Entomology, "Department of Entomology Newsletter For Alumni and Friends (2011)" (2011). Department of Entomology Newsletter. 5. http://lib.dr.iastate.edu/entnewsletter/5 This Book is brought to you for free and open access by the Entomology at Iowa State University Digital Repository. It has been accepted for inclusion in Department of Entomology Newsletter by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Sample January 2011 Newsletter For Alumni and Friends Les Lewis Retires At this writing my retirement is fast approach- ing. I always thought I would retire from my job as Research Leader and Scientist with the USDA- ARS. But things changed when Dean Wintersteen gave me the opportunity to be Chair of Entomol- ogy in the Fall of 2008 for a two-year appoint- ment. As I review my career, the common thread that runs throughout is the privilege of always being surrounded by persons that enjoyed their jobs and wanted to succeed. It has made my career enjoyable and rewarding. As I finish this appointment and decide what to do next, like many who have retired before me, I have a few things to finish from the laboratory. One scien- tific matter that I will pursue is the description of a microsporidium isolated from the western bean cutworm, Striacosta albicosta, an insect Donald Lewis presents Les Lewis with a retirement gift from the department at the holiday party in December.