Enzyme-Linked Immunosorbent Assay As a Helpful Diagnostic Tool for Pemphigus Erythematosus with Equivocal Histologic and Immunofluorescent Findings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vesiculobullous Diseases Larkin Community Hospital/NSU-COM Presenters: Yuri Kim, DO, Sam Ecker, DO, Jennifer David, DO, MBA
Vesiculobullous Diseases Larkin Community Hospital/NSU-COM Presenters: Yuri Kim, DO, Sam Ecker, DO, Jennifer David, DO, MBA Program Director: Stanley Skopit, DO, MSE, FAOCD, FAAD •We have no relevant disclosures Topics of Discussion • Subcorneal Vesiculobullous Disorders – Pemphigus foliaceous – Pemphigus erythematosus – Subcorneal pustular dermatosis (Sneddon-Wilkinson Disease) – Acute Generalized Exanthematous Pustulosis • Intraepidermal Vesiculobullous Disorders – Pemphigus vulgaris – Pemphigus vegetans – Hailey-Hailey Disease – Darier’s Disease – Grover’s Disease – Paraneoplastic Pemphigus – IgA Pemphigus Topics of Discussion (Continued) • Pauci-inflammatory Subepidermal Vesiculobullous Disorders – Porphyria Cutanea Tarda (PCT) – Epidermolysis Bullosa Acquisita (EBA) – Pemphigoid Gestationis • Inflammatory Subepidermal Disorders – Bullous Pemphigoid – Cicatricial Pemphigoid – Dermatitis Herpetiformis – Linear IgA Subcorneal Vesiculobullous Disorders • Pemphigus foliaceous • Pemphigus erythematosus • Subcorneal pustular dermatosis (Sneddon- Wilkinson Disease) • AGEP Pemphigus Foliaceous • IgG Ab to desmoglein 1 (Dsg-1, 160 kDa) • Peak onset middle age, no gender preference • Endemic form – Fogo selvagem in Brazil and other parts of South America • Pemphigus erythematosus- Localized variant of pemphigus foliaceous with features of lupus erythematosus Overview Clinical H&E DIF Treatment Pemphigus Foliaceous Overview Clinical H&E DIF Treatment Pemphigus Foliaceous Overview Clinical H&E DIF Treatment Pemphigus Foliaceous Overview Clinical -

Radiation-Induced Pemphigus Or Pemphigoid Disease in 3 Patients with Distinct Underlying Malignancies
Radiation-Induced Pemphigus or Pemphigoid Disease in 3 Patients With Distinct Underlying Malignancies Wonwoo Shon, DO; David A. Wada, MD; Amer N. Kalaaji, MD PRACTICE POINTS • The use of radiation therapy is increasing because of its therapeutic benefit, especially in advanced-stage cancer patients. • Although there is a wide range of adverse effects associated with radiation therapy, pemphigus or pemphigoid disease is rare and needs to be distinguished from other skin diseases or even recurrent underlying cancer. • The precise mechanism of radiation-induced pemphigus or pemphigoid disease is unknown, but clinicians should be alert to this potentially serious complication, and all cutaneous eruptions developing during and after radiation therapy should be evaluated with routine histologic examination in conjunction with direct immunofluorescence, serum for indirect immunofluorescence, and enzyme-linked immunosorbent assay. The cutaneous lesions of radiation-induced pem- eruption is unknown, clinicians should be alert for phigus or pemphigoid disease may resemble this potentially serious complication and evaluate other skin diseases, including recurrent underly- all cutaneous eruptions developing during and ing cancer. We performed a computerized search after radiotherapy. of Mayo Clinic (Rochester, Minnesota) archives Cutis. 2016;97:219-222. and identified 3 cases of pemphigus or pemphi- goid disease that occurredCUTIS after radiation therapy Do not copy for breast, cervical, and metastatic malignancies, number of adverse cutaneous effects may respectively. In 2 of these patients, the disease result from radiation therapy, including was initially confined to the irradiated field but A radiodermatitis, alopecia, and radiation- subsequently disseminated to other parts of the induced neoplasms. Radiation therapy rarely induces patients’ bodies, including mucosal surfaces. -

SKIN VERSUS PEMPHIGUS FOLIACEUS and the AUTOIMMUNE GANG Lara Luke, BS, RVT, Dermatology, Purdue Veterinary Teaching Hospital
VETERINARY NURSING EDUCATION SKIN VERSUS PEMPHIGUS FOLIACEUS AND THE AUTOIMMUNE GANG Lara Luke, BS, RVT, Dermatology, Purdue Veterinary Teaching Hospital This program was reviewed and approved by the AAVSB Learning Objective: After reading this article, the participant will be able to dis- RACE program for 1 hour of continuing education in jurisdictions which recognize AAVSB RACE approval. cuss and compare autoimmune diseases that have dermatological afects, includ- Please contact the AAVSB RACE program if you have any ing Pemphigus Foliaceus (PF), Pemphigus Erythematosus (PE), Discoid Lupus comments/concerns regarding this program’s validity or relevancy to the veterinary profession. Erythematosus (DLE), Systemic Lupus Erythematosus (SLE). In addition, the reader will become familiar with diagnostic and treatment techniques. FUNCTION OF THE SKIN Te skin is the largest organ of the body. Along with sensory function, it provides a barrier between the inside and outside world. Te epidermis is composed of the following fve layers: stratum basale, stratum spinosum, stratum granulosum, stratum lucidum, and stratum corneum. Te stratum lucidum is found only on the nasal planum and footpads. When the cells of the epidermis are disrupted by systemic disease, the barrier is also disrupted. Clinical signs of skin disease will bring the patient into the veterinarian’s ofce for diagnosis. 32 THE NAVTA JOURNAL | NAVTA.NET VETERINARY NURSING EDUCATION THE PEMPHIGUS COMPLEX article.3 Histologically it shares characteris- Pemphigus Foliaceus tics of both PF and DLE.1 This classifcation PF is an immune mediated pustular disor- is still considered controversial and PE may der included in a group of diseases known just be a localized variant of PF.1 as the pemphigus complex. -

(Senear-Usher Syndrome)!
ISSN Online: 2378-1726 Symbiosis www.symbiosisonlinepublishing.com Letter to the editor Clinical Research in Dermatology: Open Access Open Access Tinea Imbricate- like Pemphigus erythematosus (Senear-Usher syndrome)! Ivanka Temelkova1,2, Georgi Tchernev1,2* 1Onkoderma- Clinic for Dermatology, Venereology and Dermatologic Surgery, General Skobelev 26, 1606 Sofia. 2Medical Institute of Ministry of Interior (MVR-Sofia), Department of Dermatology, Venereology and Dermatologic Surgery Sofia, Bulgaria Received: October 29, 2019; Accepted: October 29, 2019; Published: October 30, 2019 *Corresponding author: Prof Dr. Georgi Tchernev, Onkoderma- Clinic for Dermatology, Venereology and Dermatologic Surgery, General Skobelev 26, 1606 Sofia; Medical Institute of Ministry of Interior (MVR-Sofia), Department of Dermatology, Venereology and Dermatologic Surgery Sofia, Bulgaria. E-mail: [email protected] We present a 65-year-old man with a complaint of redness and is observed. In the differential diagnostic aspect it was thought of ulceration on the scalp, as well as on the face, body and armpits, lupus erythematosus, psoriasis, mycosis, seborrheic dermatitis, worseningaccompanied in exposureby burning to andthe sun.itching He (fig.has been1-4). Complaintstreated topically date pemphigus foliaceus / erythematosus. A biopsy was taken for withback tocorticosteroids 9 months, gradually and emollients progressing, without and theeffect. patient In the observed course histological examination and direct immunofluorescence. Direct pemphigusimmunofluorescence -

An Unusual Case of Pemphigus Erythematosus
http://crcp.sciedupress.com Case Reports in Clinical Pathology 2017, Vol. 4, No. 4 CASE REPORT An unusual case of pemphigus erythematosus Dartri Cahyawari,∗ Eva K Sutedja, Unwati Sugiri, Hendra Gunawan, Oki Suwarsa Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran/Hasan Sadikin Hospital, Bandung, Indonesia Received: October 31, 2017 Accepted: December 3, 2017 Online Published: December 12, 2017 DOI: 10.5430/crcp.v4n4p14 URL: https://doi.org/10.5430/crcp.v4n4p14 ABSTRACT Pemphigus erythematosus is characterized by fragile vesicles or bullae, erosions, crusts, and scales in seborrheic area. There are several forms of atypical lesions such as erythematous papules and plaques, verrucous plaques, pustules, and lichenification. Here, we report an atypical pemphigus erythematosus with erythematous papules, plaques, and pustules skin lesions. A 52-year-old Indonesian man presented with prominent pruritic erythematous macules, papules, plaques on the scalp, trunk, and extremities, and also a pustule for each on the back and right arm. Clinically, the patient was diagnosed as small-plaque parapsoriasis, but histopathology examination on the pustule revealed a subcorneal acantholysis and direct immunofluorescence staining showed immunoglobulin G and complement C3 on the cell surface of keratinocytes. These result suitable for pemphigus erythematosus. The patient was treated with topical and systemic corticosteroid, and there were significant improvements in the skin lesions. Pemphigus erythematosus may present with -

Immunofluorescence in Dermatology
CONTINUING MEDICAL EDUCATION Immunofluorescence in dermatology Diya F. Mutasim, MD, and Brian B. Adams, MD Cincinnati, Ohio The accurate diagnosis of bullous and other immune diseases of the skin requires evaluation of clinical, histologic, and immunofluorescence findings. Immunofluorescence testing is invaluable in confirming a diagnosis that is suspected by clinical or histologic examination. This is especially true in subepidermal bullous diseases that often have overlap in the clinical and histologic findings. Direct immunofluorescence is performed on perilesional skin for patients with bullous diseases and lesional skin for patients with connective tissue diseases and vasculitis. (J Am Acad Dermatol 2001;45:803-22.) Learning objective: At the completion of this learning activity, participants should be familiar with the ideal method of obtaining immunofluorescence testing for the diagnosis of immune skin diseases and be aware of the value and limitations of immunofluorescence studies. mmunofluorescence has been used for 4 decades, both to investigate pathophysiology of Abbreviations used: skin disorders and to help physicians in the diag- I BMZ: basement membrane zone nosis of various cutaneous disorders, especially bul- BP: bullous pemphigoid lous diseases and connective tissue diseases. This CP: cicatricial pemphigoid article addresses the present status of immunofluo- DEJ: dermoepidermal junction rescence in dermatology. DH: dermatitis herpetiformis DIF: direct immunofluorescence DIAGNOSIS AND PATHOPHYSIOLOGY OF DLE: discoid lupus erythematosus BULLOUS DISEASES EBA: epidermolysis bullosa acquisita Great progress has been made during the past 5 HG: herpes gestationis decades in our understanding of the biology of the HSP: Henoch-Schönlein purpura ICS: intercellular space skin as it relates to bullous diseases. This has led to IIF: indirect immunofluorescence more accurate classification and diagnosis. -

The Molecular Aspects of Oral Mucocutaneous Diseases: a Review
International Journal of Genetics and Molecular Biology Vol. 3(10), pp. 141-148, November 2011 Available online at http://www.academicjournals.org/IJGMB DOI: 10.5897/IJGMB11.019 ISSN2006-9863 ©2011 Academic Journals Review The molecular aspects of oral mucocutaneous diseases: A review S. Sangeetha* and Dhayanand John Victor Department of Periodontics, SRM Dental College, Chennai, India. Accepted 19 October, 2011 Most of the mucocutaneous diseases are confined to the stratified squamous epithelium and thus may involve skin, oral and other mucosae like the nasal, ocular, genital mucosa. Some patients present with oral lesions only whereas in others there may be involvement of skin and other mucous membranes. An understanding of the basic molecular aspects of these disorders is essential for proper diagnosis. Once a definitive diagnosis is determined, treatment is focused upon the alleviation of clinical signs and symptoms, referral for consultation with other specialists to assess the extent of the disease process, and the prevention of recurrence. Key words: Mucocutaneous disease, desquamative gingivitis, vesiculobullous disorder, pemphigus vulgaris, pemphigoid. INTRODUCTION Mucocutaneous lesions of skin and oral mucosa manifest as desquamative gingivitis are lichen planus, commonly manifest as vesicular and/or ulcerative lesions. bullous pemphigoid (BP), pemphigus vulgaris (PV), linear Various etiological factors contribute to the development IgA disease, dermatitis herpetiformis and lupus of these lesions and encompass autoimmune/immune erythematosus. A predilection for females is generally mediated, infectious, neoplastic, hematologic, reactive, seen. nutritional and idiopathic causes (Rinaggio, 2007). An immunologic phenomenon termed epitope Approximately, 50% of oral mucosal diseases are spreading has been increasingly recognized as an localized to gingiva causing desquamative gingivitis, important pathogenic mechanism responsible for the although other intraoral and extraoral sites may be initiation and/or progression of autoimmune diseases involved. -
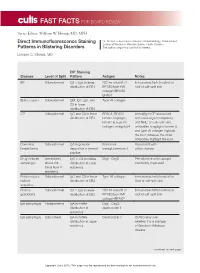
Direct Immunofluorescence Staining Patterns in Blistering Disorders
FAST FACTS FOR BOARD REVIEW Series Editor: William W. Huang, MD, MPH Direct Immunofluorescence Staining Dr. Strowd is Assistant Professor of Dermatology, Wake Forest School of Medicine, Winston-Salem, North Carolina. Patterns in Blistering Disorders The authors report no conflict of interest. Lindsay C. Strowd, MD DIF Staining Disease Level of Split Pattern Antigen Notes BP Subepidermal C3 > IgG in linear NC16a subunit of Immunoreactants located on distribution at DEJ BP180/type XVII roof of salt-split skin collagen/BPAG2, BPAG1 Bullous lupus Subepidermal IgM, IgG, IgA, and Type VII collagen C3 in linear distribution at DEJ CP Subepidermal IgG and C3 in linear BPAG1, BPAG2, Antiepiligrin CP associated distribution at DEJ laminin 5/epiligrin, with solid-organ malignancy laminin 6, type VII and NHL; on salt-split skin, collagen, integrin β4 antibodies to epiligrin/laminin 5 and type VII collagen highlight the floor, whereas the other antibodies highlight the roof Dermatitis Subepidermal IgA in granular Epidermal Associated with herpetiformis deposition in dermal transglutaminase 3 celiac disease papillae Drug-induced Immediately IgG > C3 in netlike Dsg1, Dsg3 Penicillamine and captopril pemphigus above the distribution in lower commonly implicated basal layer in epidermis epidermis Epidermolysis Subepidermal IgG and C3 in linear Type VII collagen Immunoreactants located on bullosa distribution at DEJ floor of salt-split skin acquisita Herpes Subepidermal C3 > IgG in linear NC16a subunit of Immunoreactants located on gestationis distribution at DEJ BP180/type XVII roof of salt-split skin collagen/BPAG2 IgA pemphigus Intraepidermal IgA in netlike Dsg1, Dsg3, distribution in desmocollin 1 epidermis IgA pemphigus Subcorneal IgA in netlike Desmocollin 1 Controversy over distribution in upper whether it is a subtype epidermis of Sneddon-Wilkinson disease continued on next page Copyright Cutis 2016. -

Autoimmune Blistering Disease - Diagnostic Methodology for Pemphigus and Pemphigoid
life.science.discovery. life.science.discovery. ™ Autoimmune Blistering Disease - Diagnostic Methodology for Pemphigus and Pemphigoid - Pemphigus BP EBA Epidermal side Epidermal side Dermal side Dermal side Epidermal cell-cell junction BP230 BP230 Anti-BP230 BP180 BP180 Dsg3 Anti-BP180 Anchoring fibril Anchoring fibril Dsg1 Blister Blister Anti-type VII collagen NC16a Complement www.mblintl.com Structure of the epidermis CONTENTS The epidermis generally consists of four layers: basal Normal skin Epidermal cell-cell junction layer (stratum basale), spinous layer (stratum spinosum), Structure of the epidermis 3 Desmocollin granular layer (stratum granulosum), and cornified layer Classification of autoimmune blistering diseases 3 Dsg3: desmoglein 3 (stratum corneum). Keratinocytes are the major component Epidermal cells of the epidermis. These cells progressively differentiate Pemphigus 4 from basal cells to the finally differentiated, cornified Desmosome Dsg1: desmoglein 1 Clinical characterization 4 layer, the outermost layer of the epidermis. Several Epidermal cells types of intercellular junctions in the epidermis, such as Cornified layer Pemphigus (PV/PF) and anti-desmoglein 1 & 3 IgG autoantibodies 5 Plakoglobin desmosomes and tight junctions, are involved in protection Desmoplakin Granular layer against mechanical stress, physical stimulation or infectious Plakophilin Pemphigoid 6 agents. Desmosomes are composed of transmembrane Epidermis Clinical characterization 6 proteins [e.g., desmoglein (Dsg) 1, Dsg3, and desmocollin] Dermal-epidermal junction BP230 and intracellular proteins ( , desmoplakin). Desmogleins Plectin BOX Salt-split skin immunofluorescence 7 e.g. Spinous layer and desmocollins, which are the cadherin family proteins, BP180 Basal cells Bullous pemphigoid (BP) and anti-BP180 and anti-BP230 IgG autoantibodies 8 Integrin α6β4 maintain epidermal cohesion in a Ca2+-dependent manner. -

Autoimmune Diseases in Dermatology
Ⅵ Autoimmune Diseases Autoimmune Diseases in Dermatology JMAJ 47(9): 431–435, 2004 Hiroo YOKOZEKI* and Kiyoshi NISHIOKA** *Associate Professor, **Professor, Tokyo Medical and Dental University Abstract: This article summarizes the concept, clinical characteristics, and treat- ment of bullous diseases and erythema nodosum, which are typical autoimmune diseases in the field of dermatology. Autoimmune bullous diseases are classified into the pemphigus group, with antibodies against substances between epidermal cells, and the pemphigoid group, with antibodies against epidermal basement membrane. The causative antigens for each group have recently been identified. Type-III allergic reaction induced by bacteria, medicine, etc. as causative antigens is thought to be involved in the development of erythema nodosum. The treatment of the diseases chiefly consists of removal of causative antigens and steroid hormone therapy. Key words: Pemphigus; Bullous pemphigoid; Erythema nodosum; Autoimmune disease Autoimmune Bullous Diseases and 2 show the structure and component proteins of hemidesmosome and desmosome, 1. Concept respectively. In autoimmune bullous diseases, antibodies Desmosome exists in epidermal cell mem- against epidermal antigens damage the epi- brane. Keratin intermediate-sized filaments dermis, resulting in bullous formation. Auto- combine with the intracellular adhesion plate. immune bullous diseases are divided into two Two membrane proteins of desmoglein and groups: the pemphigus group with antibodies desmocollin maintain intercellular adhesion, against substances between epidermal cells, each of which is subdivided into Types 1 to 3. and the pemphigoid group with antibodies Intracellular adhesion is based on desmo- against epidermal basement membrane. plakin (DPL), envoplakin (EPL), periplakin Desmosome and hemidesmosome are con- (PPL), placoglobin (PG), and placophilin. sidered to play important roles in the adhesion Types 1 and 3 desmoglein are the target anti- of epidermal cells. -

Vesicles & Bullae: a Review of Differential Diagnoses and Treatment
VESICLES & BULLAE: A REVIEW OF DIFFERENTIAL DIAGNOSES AND TREATMENT OPTIONS Kate Braunlich, DO, PGY4 Program Director: Dr. Richard Miller I have no relevant disclosures ◦All photos are taken from Andrews’ Diseases of the Please Note Skin Clinical Atlas unless otherwise specified. The rights/copyright to these photos remains with the authors of this text. History ◦ How long have the bullae or vesicles been present? ◦ Has the patient had bullae/vesicles before? ◦ If chronic, does the eruption occur at the same site each time? ◦ Are the bullae/vesicles symptomatic? ◦ Is the patient taking medications? If so, which medications? Physical Exam ◦ Patient age ◦ If female, childbearing status, i.e. pregnant, recently post-partum etc. ◦ Bullae/vesicle distribution ◦ Is there mucosal involvement ◦ Are the bullae/vesicles isolated or is there concomitant desquamation, erosions, fissures, scale or erythema ◦ Is there evidence of scaring Fragile or tense bullae? ◦ Fragile Bullae ◦ Tense Bullae ◦ Bullous Impetigo ◦ Contact dermatitis (allergic or irritant) ◦ Pemphigus (all variants) ◦ Bullous pemphigoid ◦ SSSS ◦ Bullous drug/fixed drug ◦ Hailey-Hailey disease ◦ Cicatricial pemphigoid/MMP ◦ DH ◦ Dyshidrotic dermatitis ◦ EBA ◦ EB ◦ EM ◦ Hand, foot, and mouth ◦ HSV/Zoster ◦ Herpes gestationis ◦ Linear IgA bullous dermatosis ◦ PCT ◦ Smallpox/Vaccinia ◦ TEN ◦ Second degree sunburn Etiologies ◦ Infectious: bacterial & viral ◦ External ◦ Autoimmune ◦ Genetic ◦ Porphyria cutanea tarda (PCT) ◦ Epidermolysis bullosa (EB) ◦ Epidermolysis bullosa acquisita (EBA) -

Pemphigus Foliaceus in an Otherwise Healthy 35-Year-Old Male Tyler Vukmer, DO,* John Hassani, DO,** Adriana Ros, DO***
Pemphigus Foliaceus in an Otherwise Healthy 35-Year-Old Male Tyler Vukmer, DO,* John Hassani, DO,** Adriana Ros, DO*** *Dermatology Resident, PGY3, Hackensack University Medical Center at Palisades, North Bergen, NJ **Hematology/Oncology Specialist, H. Lee Moffitt Cancer Center, Tampa Bay, FL ***Program Director, Dermatology Residency, Hackensack University Medical Center at Palisades, North Bergen, NJ Disclosures: None Correspondence: Tyler Vukmer, DO; [email protected] Abstract We report a classic case presentation of the rarely seen disease pemphigus foliaceus in a 35-year-old Salvadorian male with no past medical history. There is no universal gold standard for pemphigus disease treatment, and practitioners and patients should be aware that treatment may need to be adjusted during the clinical course of the disease. Immunofluorescence biopsy and antibody titers can help establish the diagnosis. We discuss the clinical features, pathophysiology, histology, disease course and treatment of pemphigus foliaceus. Introduction Pemphigus foliaceus (PF) is a rare autoimmune disorder characterized by subcorneal acantholysis mediated by IgG anti-desmoglein-1 (DSG- 1) antibodies. We encountered a case of PF in a Hispanic patient seen initially by the emergency room physician. The clinical presentation of this condition is characterized by often-thick keratotic scale on an erythematous base with neither bullae formation nor mucosal involvement, as was the case with our patient. Immunofluorescence patterns and specific antibody titers help to establish the PF diagnosis; however, the presentation may at times overlap significantly with other forms of pemphigus, so it is important for the practitioner to obtain a biopsy. There is no gold- standard algorithm for pemphigus management,1 and patients and physicians should be aware that it may be chronic and difficult to treat, requiring multiple follow- ups.