Lesion. This Was Perhaps the First Explanation That Scar Tissue
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WHAT HAPPENED? CDR, a 24-Year-Old Chinese Male
CHILDHOOD DEVELOPMENTAL SCREENING 2020 https://doi.org/10.33591/sfp.46.5.up1 FINDING A MASS WITHIN THE ORAL CAVITY: WHAT ARE THE COMMON CAUSES AND 4-7 GAINING INSIGHT: WHAT ARE THE ISSUES? In Figure 2 below, a list of masses that could arise from each site Figure 3. Most common oral masses What are the common salivary gland pathologies Salivary gland tumours (Figure 7) commonly present as channel referrals to appropriate specialists who are better HOW SHOULD A GP MANAGE THEM? of the oral cavity is given and elaborated briey. Among the that a GP should be aware of? painless growing masses which are usually benign. ey can equipped in centres to accurately diagnose and treat these Mr Tan Tai Joum, Dr Marie Stella P Cruz CDR had a slow-growing mass in the oral cavity over one year more common oral masses are: torus palatinus, torus occur in both major and minor salivary glands but are most patients, which usually involves surgical excision. but sought treatment only when he experienced a sudden acute mandibularis, pyogenic granuloma, mucocele, broma, ere are three pairs of major salivary glands (parotid, commonly found occurring in the parotid glands. e most 3) Salivary gland pathology may be primary or secondary to submandibular and sublingual) as well as hundreds of minor ABSTRACT onset of severe pain and numbness. He was fortunate to have leukoplakia and squamous cell carcinoma – photographs of common type of salivary gland tumour is the pleomorphic systemic causes. ese dierent diseases may present with not sought treatment as it had not caused any pain. -

Oral Mucocele – Diagnosis and Management
Journal of Dentistry, Medicine and Medical Sciences Vol. 2(2) pp. 26-30, November 2012 Available online http://www.interesjournals.org/JDMMS Copyright ©2012 International Research Journals Review Oral Mucocele – Diagnosis and Management Prasanna Kumar Rao 1, Divya Hegde 2, Shishir Ram Shetty 3, Laxmikanth Chatra 4 and Prashanth Shenai 5 1Associate Professor, Department of Oral Medicine and Radiology, Yenepoya Dental College, Yenepoya University, Deralakatte, Nithyanandanagar Post, Mangalore, Karnataka, India. 2Assistant Professor, Department of Obstetrics and Gynecology, AJ Institute of Medical Sciences, Mangalore, Karnataka, India. 3Reader, Department of Oral Medicine and Radiology, AB Shetty Memorial Institute of Dental Sciences, Nitte University, Mangalore, Karnataka, India. 4Senior Professor and Head, Department of Oral Medicine and Radiology, Yenepoya Dental College, Yenepoya University, Deralakatte, Nithyanandanagar Post, Mangalore, Karnataka, India. 5Senior Professor, Department of Oral Medicine and Radiology, Yenepoya Dental College, Yenepoya University, Deralakatte, Nithyanandanagar Post, Mangalore, Karnataka, India. ABSTRACT Mucocele are common salivary gland disorder which can be present in the oral cavity, appendix, gall bladder, paranasal sinuses or lacrimal sac. Common location for these lesions in oral cavity is lower lip however it also presents on other locations like tongue, buccal mucosa, soft palate, retromolar pad and lower labial mucosa. Trauma and lip biting habits are the main cause for these types of lesions. These are painless lesions which can be diagnosed clinically. In this review, a method used for searching data includes various internet sources and relevant electronic journals from the Pub Med and Medline. Keywords: Mucocels, Lower lip, Retention cyst. INTRODUCTION Mucocele is defined as a mucus filled cyst that can Types appear in the oral cavity, appendix, gall bladder, paranasal sinuses or lacrimal sac (Baurmash, 2003; Clinically there are two types, extravasation and retention Ozturk et al., 2005). -

Sublingual Epidermoid Cyst Resembling Sublingual Ranula: a Case Report
Archives of Orofacial Sciences The Journal of the School of Dental Sciences Universiti Sains Malaysia Arch Orofac Sci (2015), 10(1). Article No. 0204. 6 pages. Case Report Sublingual epidermoid cyst resembling sublingual ranula: a case report Tan Shi Nee a, Roszalina Ramli b, Primuharsa Putra Sabir Husin Athar c* a Dept. of Otorhinolarygology-Head & Neck Surgery, School of Medicine, KPJ Healthcare University College, Malaysia. b Department of Oral & Maxillofacial Surgery, Universiti Kebangsaan Malaysia. c Ear, Nose & Throat-Head & Neck Consultant Clinic, KPJ Seremban Specialist Hospital/ KPJ Healthcare University College, Negeri Sembilan, Malaysia. * Corresponding author: [email protected] Submitted: 18/11/2014. Revised edition: 23/02/2015. Accepted: 12/05/2015. Published online: 13/05/2015. Abstract Dermoid cysts are anatomic embryonic abnormalities that are rarely seen in the oral cavity. Histologically, they are further classified as epidermoid, dermoid or teratoid. We report a case in which an 18- year-old girl who developed an epidermoid cyst presenting as a large sublingual swelling occupying the entire floor of the mouth causing snoring and speech difficulty. We emphasized on the clinical steps in achieving an accurate diagnosis, possible differential diagnosis, necessary imaging techniques and management of epidermoid cyst. Keywords: enucleation, epidermoid cyst, ranula, sublingual. Introduction Dermoid cyst can be classified as congenital or acquired. A lot of Dermoid cyst is a benign cystic malformation etiopathogenesis theories have been that is rarely seen in the oral cavity (Jham et reported which includes dysontogenetic, al., 2007). It represents less than 0.01% of traumatic, and thyroglossal anomaly (Jham all oral cavity cysts (Verma et al., 2012; De et al., 2007; Verma et al., 2012). -

Guide for Dental Fees for General Dentists January 2020
Guide for Dental Fees for General Dentists January 2020 Copyright © 2019 by the Alberta Dental Association and College ALBERTA DENTAL ASSOCIATION AND COLLEGE Preamble The fees listed herein are published to serve merely as a guide. No dentist receiving this list is under any obligation to accept the fees itemized. Any dentist who does not use all or any of these fees will in no way suffer in their relations with the Alberta Dental Association and College or any other body, group or committee affiliated with or under the control of the Alberta Dental Association and College. A genuine suggested fee guide is one which is issued merely for professional information purposes without raising any intention or expectation whatsoever that the membership will adopt the guide for their practices. Dentists have the right and freedom to use any dental codes that are included in the Alberta Uniform System of Coding and List of Services. Dentists may use these fees to assist them in determining their own professional fees. A suggested protocol to follow in order to eliminate the possibility of patient misunderstandings regarding the fees for dental treatment is: a. Perform a thorough oral examination for the patient. b. Explain, carefully, the particular problems encountered in this patient's mouth. Describe your treatment plan and prognosis, in a manner, which the patient can fully understand. Assure yourself that the patient has understood the presentation. c. Present your fee for treatment, before the commencement of treatment. d. Arrange financial commitments in such a manner that the patient understands their obligation. e. -

Prevalence of Salivary Gland Disease in Patients Visiting a Private Dental
European Journal of Molecular & Clinical Medicine ISSN 2515-8260 Volume 07, Issue 01, 2020 PREVALENCE OF SALIVARY GLAND DISEASE IN PATIENTS VISITING A PRIVATE DENTAL COLLEGE 1Dr.Abarna Jawahar, 2Dr.G.Maragathavalli, 3Dr.Manjari Chaudhary 1Department of Oral Medicine and Radiology, Saveetha Dental College and Hospital, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai, India 2Professor, Department of Oral Medicine and Radiology, Saveetha Dental College and Hospital, Saveetha Institute of Medical and Technical Sciences(SIMATS), Saveetha University, Chennai, India 3Senior Lecturer, Department of Oral Medicine and Radiology, Saveetha Dental College and Hospital, Saveetha Institute of Medical and Technical Sciences(SIMATS), Saveetha University, Chennai, India [email protected] [email protected] [email protected] ABSTRACT: The aim of the study was to estimate the prevalence of salivary gland diseases in patients visiting a private dental college. A retrospective analysis was conducted on patients who visited the Department of Oral Medicine from March 2019 to March 2020.Clinically diagnosed cases of salivary gland diseases which included salivary gland neoplasms, xerostomia, necrotizing sialometaplasia, mucocele, ranula, sjogren’s syndrome, sialodochitis, sialadenitis were included in the study.The details of each case were reviewed from an electronic database.From the study we found that 17 patients were diagnosed with salivary gland disease.The most commonly observed salivary gland disease was mucocele of the lip with a frequency of 41.17% in the study population followed by xerostomia (17.65%).Salivary gland disease can occur due to variable causes and might significantly affect the quality of life and daily functioning.Only with a thorough knowledge of the subject it is possible to detect the diseases of the salivary gland in their early stage and manage them more efficiently. -

Unusual Presentation of Intralingual Dermoid Cyst with Review of the Literature
Journal of Otolaryngology-ENT Research Case Report Open Access Unusual presentation of intralingual dermoid cyst with review of the literature Abstract Volume 5 Issue 1 - 2016 We report a case of a 57-year-old female who presented with pain overlying her right Amit Bhojwani,1 Kevin Jensen,2 Jon temporomandibular joint as well as trismus. The patient was found to have a 2cm x 3cm 2 cyst within her tongue musculature on CT scan that was later diagnosed as an intralingual Robitschek 1Department of Otolaryngology and Facial Plastic Surgery, dermoid cyst by pathology. These entities are exceedingly rare in the head and neck. A Rowan University School of Osteopathic Medicine, USA transoral midline glossotomy approach was undertaken to completely excise the cyst 2Department of Otolaryngology and Facial Plastic Surgery, Joint without complication and she had an uneventful recovery without recurrence of her cyst. Base Elmendorf- Richardson, USA This case is unique in multiple ways. First, the patient was close to 60years of age. Most nd rd of these patients are in their 2 -3 decade of life. Most dermoid cysts are found in the Correspondence: Amit Bhojwani, Department of sublingual, submental space, or submandibular spaces, which is unlike our patient, who Otolaryngology and Facial Plastic Surgery, Rowan University was found to have an intralingual cyst. These patients classically present with dyspnea, School of Osteopathic Medicine, 2 East Laurel Road, Ste 2600, dysphagia, or dysphonia. Lastly, the patient presented with right TMJ pain and trismus, Stratford, NJ 08084, USA, Email which were not related to the cyst itself. Thus, the cyst was an incidental finding on routine imaging. -

Classic Approaches to Sialoendoscopy for Treatment of Sialolithiasis ODED NAHLIELI
7 Classic Approaches to Sialoendoscopy for Treatment of Sialolithiasis ODED NAHLIELI Obstructive sialadenitis, with or without sialolithiasis, sialoadenitis. These data do not include patients who represents the main inflammatory disorder of the major were treated as ambulatory (outpatient) cases. salivary glands. The diagnosis and treatment of obstruc- There is a male preponderance,5 and the peak tions and inflammations of these glands can be proble- incidence is between the ages of 30 and 60.5 Sialoliths matic due to the limitations of standard imaging grow by deposition and range in size from 0.1 to techniques. Satisfactory treatment depends on our 30 mm.6 Presentation is typically with a painful swelling ability to reach a precise diagnosis and, in the case of of the gland at meal times, when the obstruction caused sialoliths, to accurately locate the obstruction. Until by the calculus becomes most acute.7 recently many of these glands required complete During the past decade, with the introduction of removal under general anesthesia. salivary gland endoscopy there has been a major step Sialolithiasis is a common finding, accounting for forward, not only in providing an accurate means of 50% of major salivary gland disease.1,2 The subman- diagnosing and locating intraductal obstructions, but dibular gland is the most prone to sialolithiasis. In also in permitting minimally invasive surgical treatment various studies it was found that Â/80% of all sialo- that can successfully manage those blockages that are lithiasis cases are in the submandibular glands, 19% not accessible intraorally.8 Á20 occur in the parotid gland, and Â/1% are found in the sublingual gland. -

EVALUATION of ASSOCIATION BETWEEN IMPACTED TEETH and TEMPOROMANDIBULAR JOINT DISORDERS Trishala A1 , M.P.Santhosh Kumar2 , Arthi B3
European Journal of Molecular & Clinical Medicine ISSN 2515-8260 Volume 07, Issue 01, 2020 EVALUATION OF ASSOCIATION BETWEEN IMPACTED TEETH AND TEMPOROMANDIBULAR JOINT DISORDERS Trishala A1 , M.P.Santhosh Kumar2 , Arthi B3 1Saveetha Dental College and Hospitals Saveetha Institute of Medical and Technical Sciences Saveetha University Chennai, India 2Reader Department of Oral and Maxillofacial SurgerySaveetha Dental College and Hospitals Saveetha Institute of Medical and Technical Sciences Saveetha University Chennai, India 3Associate Professor Department of Public Health Dentistry Saveetha Dental College and Hospitals Saveetha Institute of Medical and Technical Sciences Saveetha University Chennai, India [email protected] [email protected] [email protected] ABSTRACT Impaction of the third molars has been established as a factor with the potential to damage temporomandibular joints. Furthermore, the trauma resulting from the surgery of third molars has been reported to be a predisposing factor in the progression of temporomandibular joint disorders (TMD) symptoms. The high frequency of third molar surgery can result in an increased number of patients who suffer from chronic oral and facial pains. Thus, it is important to identify those patients who have pre‐existing pain or any signs of dysfunction in their temporomandibular joints and masticatory structures, prior to third molar surgery. The aim of this study was to evaluate the association between impacted teeth and temporomandibular joint disorders. A retrospective study was conducted by reviewing the case records of patients who underwent treatment in Saveetha Dental College and Hospital from June 2019 - March 2020. The study population included 96 patients diagnosed with temporomandibular joint disorders and 98 patients without TMD. -
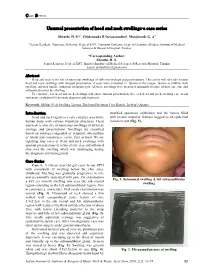
Unusual Presentation of Head and Neck Swellings-A Case Series
Case Series Unusual presentation of head and neck swellings-a case series Shruthi. H. S.1,*, Chidananda R Devasamudra2, Manjunath. G. A3 1Senior Resident, 2Assoicate Professor, Dept. of ENT, 3Assistant Professor, Dept. of Dentistry, Shridevi Institute of Medical Sciences & Research Hospital, Tumkur *Corresponding Author: Shruthi. H. S. Senior Resident, Dept. of ENT, Shridevi Institute of Medical Sciences & Research Hospital, Tumkur Email: [email protected] Abstract Head and neck is the site of numerous swellings of different etiology and presentations. This article will describe various head and neck swellings with unusual presentation. 4 cases were evaluated i.e. lipoma of the tongue, lipoma as midline neck swelling, infected ranula, epidermal inclusion cyst. All these swellings were presented unusually because of their site, size and inflamed skin over the swelling. To conclude, few head and neck swellings will show unusual presentation. So, each head and neck swelling case needs systematic evaluation for accurate diagnosis and treatment. Keywords: Midline Neck Swelling, Lipoma, Epidermal Inclusion Cyst, Ranula, Ludwig’s Angina. Introduction stratified squamous epithelium and the lumen filled Head and neck region is a very complex area in the with keratin material, features suggestive of epidermal human body with various important structures. Head inclusion cyst (Fig. 5). and neck is also site of numerous swellings of different etiology and presentations. Swellings are classified based on etiology-congenital or acquired, site-midline or lateral and consistency- cystic, firm or hard. We are reporting four cases of Head and neck swellings with unusual presentations in terms of site, size and inflamed skin over the swelling which was challenging during the diagnosis and management. -

A Guide to Salivary Gland Disorders the Salivary Glands May Be Affected by a Wide Range of Neoplastic and Inflammatory
MedicineToday PEER REVIEWED ARTICLE CPD 1 POINT A guide to salivary gland disorders The salivary glands may be affected by a wide range of neoplastic and inflammatory disorders. This article reviews the common salivary gland disorders encountered in general practice. RON BOVA The salivary glands include the parotid glands, examination are often adequate to recognise and MB BS, MS, FRACS submandibular glands and sublingual glands differentiate many of these conditions. A wide (Figure 1). There are also hundreds of minor sali- array of benign and malignant neoplasms may also Dr Bova is an ENT, Head and vary glands located in the mucosa of the hard and affect the salivary glands and a neoplasia should Neck Surgeon, St Vincent’s soft palate, oral cavity, lips, tongue and oro - always be considered when assessing a salivary Hospital, Sydney, NSW. pharynx. The parotid gland lies in the preauricular gland mass. region and extends inferiorly over the angle of the mandible. The parotid duct courses anteriorly Inflammatory disorders from the parotid gland and enters the mouth Acute sialadenitis through the buccal mucosa adjacent to the second Acute inflammation of the salivary glands is usu- upper molar tooth. The submandibular gland lies ally of viral or bacterial origin. Mumps is the most in the submandibular triangle and its duct passes common causative viral illness, typically affecting anteriorly along the floor of the mouth to enter the parotid glands bilaterally. Children are most adjacent to the frenulum of the tongue. The sub- often affected, with peak incidence occurring at lingual glands are small glands that lie just beneath approximately 4 to 6 years of age. -

Oral Medicine Case Book 73: HIV Associated Oral Ulcerations: Differential Diagnosis
128 > CASE BOOK Oral medicine case book 73: HIV associated oral ulcerations: differential diagnosis SADJ April 2016, Vol 71 no 3 p128 - p130 T Kungoane1, JC Marnewick2, WFP van Heerden3 CASE REPORT Clinical Presentation ACRONYMS A 36 year old female suffering from multiple oral ulcers ARV: antiretroviral treatment was referred to the Department of Periodontics and Oral CMV: cytomegalovirus Medicine clinic at the University of Pretoria in February EBV: Epstein-Barr virus 2016. The ulcers had been present for a month, were first seen as “small sores” that had subsequently increased large immunoblast-like cells with large nuclei, prominent in size. On examination, multiple, relatively painful, large nucleoli and ample cytoplasm. Viral cytopathic effects (>1cm) ulcers were seen on the palate, labial mucosa, were not seen. Following routine histology examination, tongue and gingiva (Figure 1). She also had gingivitis and a special stains were ordered to exclude an infectious plunging ranula of the submandibular gland and reported aetiology (Table 1). Immunohistochemical stains and in-situ recent weight loss. The patient had been diagnosed with hybridization for EBV-encoded RNAs (EBER) were done retroviral disease in 2010; her latest CD4 count was 49 to exclude viral and neoplastic infiltrates. The antibodies with a viral load of 111 797 copies. Her haemoglobin (Hb) used, the source, clone and dilution together with results was 11. 7g/dL and the estimated glomerular filtration are summarised in Table 2. The final diagnosis was that rate (eGFR) >60mL/min. She had defaulted antiretroviral of HIV- associated oral major aphthous ulceration. The treatment (ARV) for a year and was reinstated on patient was subsequently given chlorhexidine gluconate treatment, on ARVs regimen 2 comprising of Lamzid and mouthwash and metronidazole. -

Acute Salivary Gland Inflammation Associated with Systemic Lupus Erythematosus
Ann Rheum Dis: first published as 10.1136/ard.31.5.384 on 1 September 1972. Downloaded from Ann. rheum. Dis. (1792), 31, 384 Acute salivary gland inflammation associated with systemic lupus erythematosus W. A. KATZ AND G. E. EHRLICH The Arthritis Center, Albert Einstein Medical Center-Moss Rehabilitation Hospital; Temple University School ofMedicine, Philadelphia, Pennsylvania, U.S.A. The parotid gland may enlarge during the course of her physician, even though there had been no known systemic lupus erythematosus (SLE). Shearn and exposure to infection. The symptoms disappeared within Pirofsky (1952) were the first to draw attention to this a few days; however, the patient subsequently became studying a group of 34 patients aware of exertional dyspnoea and pruritic papular erup- association while tions on the flexor surfaces of the extremities and face. who had systemic lupus erythematosus. They dis- Physical examination showed multiple pigmented covered sialoadenitis characterized by chronic, non- macular areas covering the arms, legs, and malar region. suppurative inflammation unilaterally in one and There was clinical evidence of pericardial and pleural bilaterally in two patients. Morgan (1954) reported effusions, and tenderness was present in the right and left systemic lupus erythematosus in a patient with the costovertebral angles. The liver, spleen, and kidneys were copyright. Sjogren-Mikulicz syndrome, and Harvey, Shulman, not palpable. The salivary glands were not enlarged or Tumulty, Conley, and Schoenrich (1954) described a tender. There was a synovitis of both wrists. 13-year-old Negro girl who developedchronic parotid Chest x rays confirmed the existance of a small left and died from classical lupus pleural effusion with moderate globular cardiomegaly.