Insect Derived Extra Oral GH32 Plays a Role in Susceptibility of Wheat to Hessian Fy Subhashree Subramanyam1,2*, Jill A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Parasitoid Complex Associated with the Invasive Swede Midge
The parasitoid complex associated with the invasive swede midge, Contarinia nasturtii Kieffer (Diptera: Cecidomyiidae) in Europe: prospects for classical biological control in North America. By Paul K. Abram A thesis submitted to the Faculty of Graduate Studies and Research in partial fiilfillment of the requirements for the degree of Master of Science in Biology Carleton University, Ottawa, Ontario, Canada ©2012, Paul K. Abram Library and Archives Bibliotheque et Canada Archives Canada Published Heritage Direction du Branch Patrimoine de I'edition 395 Wellington Street 395, rue Wellington Ottawa ON K1A0N4 Ottawa ON K1A 0N4 Canada Canada Your file Votre reference ISBN: 978-0-494-87830-9 Our file Notre reference ISBN: 978-0-494-87830-9 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing Library and permettant a la Bibliotheque et Archives Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par telecommunication ou par I'lnternet, preter, telecommunication or on the Internet, distribuer et vendre des theses partout dans le loan, distrbute and sell theses monde, a des fins commerciales ou autres, sur worldwide, for commercial or non support microforme, papier, electronique et/ou commercial purposes, in microform, autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve la propriete du droit d'auteur ownership and moral rights in this et des droits moraux qui protege cette these. Ni thesis. Neither the thesis nor la these ni des extraits substantiels de celle-ci substantial extracts from it may be ne doivent etre imprimes ou autrement printed or otherwise reproduced reproduits sans son autorisation. -

Bulletin Number / Numéro 2 Entomological Society of Canada Société D’Entomologie Du Canada June / Juin 2008
Volume 40 Bulletin Number / numéro 2 Entomological Society of Canada Société d’entomologie du Canada June / juin 2008 Published quarterly by the Entomological Society of Canada Publication trimestrielle par la Société d’entomologie du Canada ............................................................... .................................................................................................................................................................................................................................................................................................................................. .......................................................................... ........................................................................................................................................................................ ....................... ................................................................................. ................................................. List of contents / Table des matières Volume 40 (2), June / june 2008 Up front / Avant-propos ................................................................................................................49 Moth balls / Boules à mites .............................................................................................................51 Meeting announcements / Réunions futures ..................................................................................52 Dear Buggy / Cher Bibitte ..............................................................................................................53 -

Economic Cost of Invasive Non-Native Species on Great Britain F
The Economic Cost of Invasive Non-Native Species on Great Britain F. Williams, R. Eschen, A. Harris, D. Djeddour, C. Pratt, R.S. Shaw, S. Varia, J. Lamontagne-Godwin, S.E. Thomas, S.T. Murphy CAB/001/09 November 2010 www.cabi.org 1 KNOWLEDGE FOR LIFE The Economic Cost of Invasive Non-Native Species on Great Britain Acknowledgements This report would not have been possible without the input of many people from Great Britain and abroad. We thank all the people who have taken the time to respond to the questionnaire or to provide information over the phone or otherwise. Front Cover Photo – Courtesy of T. Renals Sponsors The Scottish Government Department of Environment, Food and Rural Affairs, UK Government Department for the Economy and Transport, Welsh Assembly Government FE Williams, R Eschen, A Harris, DH Djeddour, CF Pratt, RS Shaw, S Varia, JD Lamontagne-Godwin, SE Thomas, ST Murphy CABI Head Office Nosworthy Way Wallingford OX10 8DE UK and CABI Europe - UK Bakeham Lane Egham Surrey TW20 9TY UK CABI Project No. VM10066 2 The Economic Cost of Invasive Non-Native Species on Great Britain Executive Summary The impact of Invasive Non-Native Species (INNS) can be manifold, ranging from loss of crops, damaged buildings, and additional production costs to the loss of livelihoods and ecosystem services. INNS are increasingly abundant in Great Britain and in Europe generally and their impact is rising. Hence, INNS are the subject of considerable concern in Great Britain, prompting the development of a Non-Native Species Strategy and the formation of the GB Non-Native Species Programme Board and Secretariat. -

Contarinia Nasturtii
EXOTIC PEST FACT SHEET 11 Swede Midge (Contarinia nasturtii) What are the main hosts? How do they spread? How can I protect my industry? Swede midge is a serious threat to cabbage, broccoli, The Swede midge is a relatively weak flyer and most Check your production sites frequently for the presence cauliflower, Brussels sprouts and other crucifers. Organic easily spreads to new areas in infested transplant of new diseases and unusual symptoms. Make sure you growers may be at greater risk because they lack material. It will also appear near infested crucifer crops are familiar with common pests and diseases of your effective plant protection options. from the previous season, especially if new crops are industry so you can recognise something different. planted downwind. Infestation may be reduced with What do they look like? rotation to non-crucifer crops every 2-3 years. Swede midges spend the winter as pupae in the top 1-5 cm of Adults have a 1.5-2 mm long body (Fig 1). Eggs are laid the soil. As the temperature becomes favourable, adults in clusters of 2-50 eggs, primarily on the newest growth emerge and make their way to the soil surface. points of plants and leaf axils. Each female will lay about 100 eggs during her short (1-5 day) lifetime. Eggs are Fig 2. Swede midge very small (0.3 mm) and transparent at first, but change Where are they present? larva to a creamy white colour as they mature. Swede midge is native to Europe and southwest Asia. It Image: Susan Ellis, USDA APHIS PPQ, Bugwood.org. -

Yolanda H. Chen, Ph.D. Associate Professor of Agroecology of Specialty Crops Department of Plant and Soil Sciences University of Vermont
Yolanda H. Chen, Ph.D. Associate Professor of Agroecology of Specialty Crops Department of Plant and Soil Sciences University of Vermont EXTENDED ACADEMIC CURRICULUM VITAE Table of Contents I. Education ............................................................................................................................ 1 II. Professional Experience ...................................................................................................... 1 III. Teaching and Training Experience ...................................................................................... 2 IV. Funding ............................................................................................................................... 3 V. Publications ......................................................................................................................... 6 VI. Presentations .................................................................................................................... 11 VII. Publicity…………………………………………………………………………………………………………….……......18 VIII. Academic and Professional Service .................................................................................. 19 IX. Additional Skills ................................................................................................................. 16 X. Graduate and undergraduate student advisees………………………………………………………………………………………………….….………………..21 Yolanda H. Chen Department of Plant & Soil Science 63 Carrigan Drive University of Vermont Burlington, VT 05401, USA (802) -

Hessian Fly, Mayetiola Destructor (Diptera: Cecidomyiidae), Smart-Trap Design and Deployment Strategies
Hessian fly, Mayetiola destructor (Diptera: Cecidomyiidae), smart-trap design and deployment strategies by Ryan B. Schmid B.S., South Dakota State University, 2011 M.S., South Dakota State University, 2014 AN ABSTRACT OF A DISSERTATION submitted in partial fulfillment of the requirements for the degree DOCTOR OF PHILOSOPHY Department of Entomology College of Agriculture KANSAS STATE UNIVERSITY Manhattan, Kansas 2018 Abstract Timely enactment of insect pest management and incursion mitigation protocols requires development of time-sensitive monitoring approaches. Numerous passive monitoring methods exist (e.g., insect traps), which offer an efficient solution to monitoring for pests across large geographic regions. However, given the number of different monitoring tools, from specific (e.g., pheromone lures) to general (e.g., sticky cards), there is a need to develop protocols for deploying methods to effectively and efficiently monitor for a multitude of potential pests. The non-random movement of the Hessian fly, Mayetiola destructor (Say) (Diptera: Cecidomyiidae), toward several visual, chemical, and tactile cues, makes it a suitable study organism to examine new sensor technologies and deployment strategies that can be tailored for monitoring specific pests. Therefore, the objective was to understand Hessian fly behavior toward new sensor technologies (i.e., light emitting diodes (LEDs) and laser displays) to develop monitoring and deployment strategies. A series of laboratory experiments and trials were conducted to understand how -

Hessian Fly CP
INDUSTRY BIOSECURITY PLAN For the Grains Industry Threat-Specific Contingency Plan COMMON NAME: Hessian fly SCIENTIFIC NAME: Mayetiola destructor (Say) 1817 Cecidomyia contractor; Cecidomyia culmicola Morris 1849; SYNONYMS: Cecidomyia destructor Say 1817; Cecidomyia frumentaria Rondani 1864; Chortomyia secalina (Loew 1858); Mayetiola secalis Bollow 1950; Phytophaga cerealis Rondani 1843; Phytophaga destructor (Say 1817); Mayetiola mimeuri (Mesnil 1934) The scientific and technical content of this document is current to the date published and all efforts were made to obtain relevant and published information on the pest. New information will be included as it becomes available, or when the document is reviewed. The material contained in this publication is produced for general information only. It is not intended as professional advice on any particular matter. No person should act or fail to act on the basis of any material contained in this publication without first obtaining specific, independent professional advice. Plant Health Australia and all persons acting for Plant Health Australia in preparing this publication, expressly disclaim all and any liability to any persons in respect of anything done by any such person in reliance, whether in whole or in part, on this publication. The views expressed in this publication are not necessarily those of Plant Health Australia. Contacts: John Botha* [email protected], Andy Szito [email protected] or Darryl Hardie [email protected] Department of Agriculture, Bentley Delivery Centre, WA, 6983. *Tel. (08) 93683755 Background General M. destructor is a species of European origin accidentally introduced into North America in about 1776, and into New Zealand by 1888 Host range The information below is mainly out of the Crop Protection Compendium (On-line version, 2005): Primary hosts: Triticum spp. -
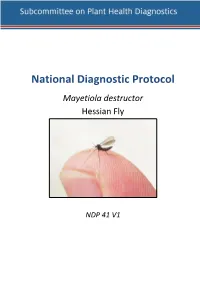
NDP 41 Hessian
NDP 41 V1- National Diagnostic Protocol for Mayetiola destructor National Diagnostic Protocol Mayetiola destructor Hessian Fly NDP 41 V1 NDP 41 V1 - National Diagnostic Protocol for Mayetiola destructor © Commonwealth of Australia Ownership of intellectual property rights Unless otherwise noted, copyright (and any other intellectual property rights, if any) in this publication is owned by the Commonwealth of Australia (referred to as the Commonwealth). Creative Commons licence All material in this publication is licensed under a Creative Commons Attribution 3.0 Australia Licence, save for content supplied by third parties, logos and the Commonwealth Coat of Arms. Creative Commons Attribution 3.0 Australia Licence is a standard form licence agreement that allows you to copy, distribute, transmit and adapt this publication provided you attribute the work. A summary of the licence terms is available from http://creativecommons.org/licenses/by/3.0/au/deed.en. The full licence terms are available from https://creativecommons.org/licenses/by/3.0/au/legalcode. This publication (and any material sourced from it) should be attributed as: Subcommittee on Plant Health Diagnostics (2018). National Diagnostic Protocol for Mayetiola destructor – NDP41 V1. (Eds. Subcommittee on Plant Health Diagnostics) Authors Severtson, D, Szito, A.; Reviewers Nicholas, A, Kehoe, M. ISBN 978-0-6481143-3-8 CC BY 3.0. Cataloguing data Subcommittee on Plant Health Diagnostics (2018). National Diagnostic Protocol for Mayetiola destructor – NDP41 V1. (Eds. Subcommittee -

Invasive Plant Mapping Protocols
For more information: [email protected] Invasive Species Mapping Protocols Objective The objective for creating these mapping protocols is to develop a unified database of invasive species occurrences within the Midwest region of the United States. These protocols were created in partnership with the Michigan Invasive Species Coalition (MISC). These guidelines will assist all partners within the region in documenting the occurrence and spread of invasive species. The data collected will allow for the development and implementation of effective control strategies in the region. Documentation: All information about invasive species occurrences must be documented using the categories on the official data form. All locations must be recorded using GPS. New occurrences should be marked with flagging ONLY if necessary to help relocate the invasive species for treatment. Volunteers must obtain permission before using flagging. The use of flagging or other references should be noted on the data form under the comments section. All equipment, materials and instructions will be provided by project partners as needed. GPS Protocols: For all observations, record a point location in the center of each population. If the area is heavily infested and distinct populations can be easily seen one to the next, mark no more than ONE point per 100ft. An average of 25 points should be taken per waypoint. All GPS points must be labeled with a unique ID number using the numbering convention explained below. Unique ID: For each GPS position collected, record a unique twelve or thirteen digit number (depending on the invasive species code) in the GPS unit for each point as follows: “BBJJGM00141U” where BB is the two letter code for the natural area, JJ is the mapper’s initials, GM is the invasive species code, 001 is the three digit sequence number, 4 is the area, 1 is the sparse density, and U is for untreated. -

Citation: Badenes-Pérez, F. R. 2019. Trap Crops and Insectary Plants in the Order 2 Brassicales
1 Citation: Badenes-Pérez, F. R. 2019. Trap Crops and Insectary Plants in the Order 2 Brassicales. Annals of the Entomological Society of America 112: 318-329. 3 https://doi.org/10.1093/aesa/say043 4 5 6 Trap Crops and Insectary Plants in the Order Brassicales 7 Francisco Rubén Badenes-Perez 8 Instituto de Ciencias Agrarias, Consejo Superior de Investigaciones Científicas, 28006 9 Madrid, Spain 10 E-mail: [email protected] 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 ABSTRACT This paper reviews the most important cases of trap crops and insectary 26 plants in the order Brassicales. Most trap crops in the order Brassicales target insects that 27 are specialist in plants belonging to this order, such as the diamondback moth, Plutella 28 xylostella L. (Lepidoptera: Plutellidae), the pollen beetle, Meligethes aeneus Fabricius 29 (Coleoptera: Nitidulidae), and flea beetles inthe genera Phyllotreta Psylliodes 30 (Coleoptera: Chrysomelidae). In most cases, the mode of action of these trap crops is the 31 preferential attraction of the insect pest for the trap crop located next to the main crop. 32 With one exception, these trap crops in the order Brassicales have been used with 33 brassicaceous crops. Insectary plants in the order Brassicales attract a wide variety of 34 natural enemies, but most studies focus on their effect on aphidofagous hoverflies and 35 parasitoids. The parasitoids benefiting from insectary plants in the order Brassicales 36 target insects pests ranging from specialists, such as P. xylostella, to highly polyfagous, 37 such as the stink bugs Euschistus conspersus Uhler and Thyanta pallidovirens Stål 38 (Hemiptera: Pentatomidae). -

Brassica Spp.) – 151
II.3. BRASSICA CROPS (BRASSICA SPP.) – 151 Chapter 3. Brassica crops (Brassica spp.) This chapter deals with the biology of Brassica species which comprise oilseed rape, turnip rape, mustards, cabbages and other oilseed crops. The chapter contains information for use during the risk/safety regulatory assessment of genetically engineered varieties intended to be grown in the environment (biosafety). It includes elements of taxonomy for a range of Brassica species, their centres of origin and distribution, reproductive biology, genetics, hybridisation and introgression, crop production, interactions with other organisms, pests and pathogens, breeding methods and biotechnological developments, and an annex on common pathogens and pests. The OECD gratefully acknowledges the contribution of Dr. R.K. Downey (Canada), the primary author, without whom this chapter could not have been written. The chapter was prepared by the OECD Working Group on the Harmonisation of Regulatory Oversight in Biotechnology, with Canada as the lead country. It updates and completes the original publication on the biology of Brassica napus issued in 1997, and was initially issued in December 2012. Data from USDA Foreign Agricultural Service and FAOSTAT have been updated. SAFETY ASSESSMENT OF TRANSGENIC ORGANISMS: OECD CONSENSUS DOCUMENTS, VOLUME 5 © OECD 2016 152 – II.3. BRASSICA CROPS (BRASSICA SPP.) Introduction The plants within the family Brassicaceae constitute one of the world’s most economically important plant groups. They range from noxious weeds to leaf and root vegetables to oilseed and condiment crops. The cole vegetables are perhaps the best known group. Indeed, the Brassica vegetables are a dietary staple in every part of the world with the possible exception of the tropics. -

Responses of the Swede Midge, Contarinia Nasturtii, to Volatile Compounds from Arabidopsis Thaliana and Brassica Oleracea Var
Master project in the Horticultural Science Programme 2008-02, 20 p (30 ECTS) Responses of the swede midge, Contarinia nasturtii, to volatile compounds from Arabidopsis thaliana and Brassica oleracea var. botrytis Photo: Linda-Marie Rännbäck by Johannes Albertsson Fakulteten för landskapsplanering, trädgårds- och jordbruksvetenskap SLU-Alnarp PROJECT INFORMATION This report is a result of a master thesis (30 ects) carried out at the department of Plant Protection Biology within the Horticultural programme at the Swedish University of Agricultural Sciences. Tina Boddum and Ylva Hillbur were supervisors and Peter Anderson was the examiner. 2 ABSTRACT The swede midge, Contarinia nasturtii, is a serious gall-forming insect pest of most cultivated Brassicaceae such as cauliflower and cabbage. Arabidopsis thaliana, also a Brassicaceae, is a well-known model plant for which the genome is sequenced and several well-characterized mutants and transgenic genotypes are available. Previous results indicate that odours from cauliflower attract C. nasturtii. In this study the responses of C. nasturtii to volatiles from cauliflower and Arabidopsis were tested in a four-arm and a y-tube olfactometer. The results indicate that females are attracted to volatiles both from cauliflower and Arabidopsis. Males on the other hand were not attracted. This study also suggests improvements for the y-tube as well as a four-arm olfactometer setup. SAMMANFATTNING Kålgallmyggan, Contarinia nasturtii, är en skadegörare på flertalet odlade grödor tillhörande familjen Brassicaceae så som blomkål, vitkål och broccoli. Arabidopsis thaliana, som också tillhör denna familj, är en viktig modellväxt eftersom dess genom helt är kartlagt och att en mängd väldokumenterade mutationer och transgena genotyper finns tillgängliga.