Absolute Configuration at the Α Carbon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

II. Stereochemistry 5
B.Sc.(H) Chemistry Semester - II Core Course - III (CC-III) Organic Chemistry - I II. Stereochemistry 5. Physical and Chemical Properties of Stereoisomers Dr. Rajeev Ranjan University Department of Chemistry Dr. Shyama Prasad Mukherjee University, Ranchi 1 Syllabus & Coverage Syllabus II Stereochemistry: Fischer Projection, Newmann and Sawhorse Projection formulae and their interconversions. Geometrical isomerism: cis–trans and syn-anti isomerism, E/Z notations with Cahn Ingold and Prelog (CIP) rules for determining absolute configuration. Optical Isomerism: Optical Activity, Specific Rotation, Chirality/Asymmetry, Enantiomers, Molecules with two or more chiral-centres, Distereoisomers, Meso structures, Racemic mixture. Resolution of Racemic mixtures. Relative and absolute configuration: D/L and R/S designations. Coverage: 1. Types of Isomers : Comparing Structures 2. Optical Activity 3. Racemic Mixtures : Separation of Racemic Mixtures 4. Enantiomeric Excess and Optical Purity 5. Relative and Absolute Configuration 6. Physical and Chemical Properties of Stereoisomers 2 Stereochemistry Types of Isomers Dr. Rajeev Ranjan 3 Stereochemistry Determining the Relationship Between Two Non-Identical Molecules Dr. Rajeev Ranjan 4 Stereochemistry Comparing Structures: Are the structures connected the same? yes no Are they mirror images? Constitutional Isomers yes no Enantiomers Enantiomers Is there a plane of symmetry? All chiral centers will be opposite between them. yes no Meso Diastereomers superimposable Dr. Rajeev Ranjan 5 Stereochemistry Optical Activity: • The chemical and physical properties of two enantiomers are identical except in their interaction with chiral substances. • The physical property that differs is the behavior when subjected to plane-polarized light ( this physical property is often called an optical property). • Plane-polarized (polarized) light is light that has an electric vector that oscillates in a single plane. -

Demonstration of Imino Acids As Products of the Reactions Catalyzed by D- and L-Amino Acid Oxidases
Proc. Nat. Acad. Sci. USA Vol. 68, No. ;i, pp. 987-991, Mlay 1971 Demonstration of Imino Acids as Products of the Reactions Catalyzed by D- and L-Amino Acid Oxidases EDMUND W. HAFNER AND DANIEL WELLNER Department of Biochemistry, Cornell University Medical College, New York, N.Y. 10021 Communicated by Alton Meister, February 23, 1971 ABSTRACT It had long been thought, but never dem- from the enzyme as the primary oxidation product. Other in- onstrated, that imino acids are formed in the reactions terpretations, however, are not (see catalyzed by D- and L-amino acid oxidases (EC 1.4.3.3 and excluded Discussion be- 1.4.3.2). The formation of imino acids is now shown low). directly by allowing the amino acid oxidase reaction to Coffey, Neims, and Hellerman (9) recently observed that proceed in the presence of NaBH4, when the imino acid is when a mixture of D-amino acid oxidase and ['4C]D-alanine reduced to the corresponding racemic amino acid. Thus, was treated with sodium borohydride, the alanine moiety when NaBH4 is added to a mixture of D-amino acid oxidase and D-alanine, a significant amount of L-alanine is formed. became covalently attached to the enzyme; they subsequently Analogous results are obtained using L-amino acid oxidase found that the labeled protein gave e-N-(1-carboxyethyl)-L- and L-leucine. Since D-amino acid oxidase is active in the lysine on hydrolysis (10). Studies in this laboratory have con- presence of NaBH4, L-alanine continues to be formed un- firmed this finding and have also shown that i- and D-amino til most of the D-isomer is oxidized by the enzyme. -

Enantiomeric Resolution and Absolute Configuration of a Chiral Δ-Lactam
molecules Article Enantiomeric Resolution and Absolute Configuration of a Chiral δ-Lactam, Useful Intermediate for the Synthesis of Bioactive Compounds 1, 1, 1 1 Roberta Listro y, Giacomo Rossino y, Serena Della Volpe , Rita Stabile , Massimo Boiocchi 2 , Lorenzo Malavasi 3, Daniela Rossi 1,* and Simona Collina 1 1 Department of Drug Sciences, University of Pavia, via Taramelli 12, 27100 Pavia, Italy; [email protected] (R.L.); [email protected] (G.R.); [email protected] (S.D.V.); [email protected] (R.S.); [email protected] (S.C.) 2 Centro Grandi Strumenti, University of Pavia, via Bassi 21, 27100 Pavia, Italy; [email protected] 3 Department of Chemistry, University of Pavia, via Taramelli 12, 27100 Pavia, Italy; [email protected] * Correspondence: [email protected] These authors contributed equally to this work. y Academic Editor: Józef Drabowicz Received: 2 December 2020; Accepted: 18 December 2020; Published: 19 December 2020 Abstract: During the past several years, the frequency of discovery of new molecular entities based on γ- or δ-lactam scaffolds has increased continuously. Most of them are characterized by the presence of at least one chiral center. Herein, we present the preparation, isolation and the absolute configuration assignment of enantiomeric 2-(4-bromophenyl)-1-isobutyl-6-oxopiperidin-3-carboxylic acid (trans-1). For the preparation of racemic trans-1, the Castagnoli-Cushman reaction was employed. (Semi)-preparative enantioselective HPLC allowed to obtain enantiomerically pure trans-1 whose absolute configuration was assigned by X-ray diffractometry. Compound (+)-(2R,3R)-1 represents a reference compound for the configurational study of structurally related lactams. -

Amino Acid Transport Pathways in the Small Intestine of the Neonatal Rat
Pediat. Res. 6: 713-719 (1972) Amino acid neonate intestine transport, amino acid Amino Acid Transport Pathways in the Small Intestine of the Neonatal Rat J. F. FITZGERALD1431, S. REISER, AND P. A. CHRISTIANSEN Departments of Pediatrics, Medicine, and Biochemistry, and Gastrointestinal Research Laboratory, Indiana University School of Medicine and Veterans Administration Hospital, Indianapolis, Indiana, USA Extract The activity of amino acid transport pathways in the small intestine of the 2-day-old rat was investigated. Transport was determined by measuring the uptake of 1 mM con- centrations of various amino acids by intestinal segments after a 5- or 10-min incuba- tion and it was expressed as intracellular accumulation. The neutral amino acid transport pathway was well developed with intracellular accumulation values for leucine, isoleucine, valine, methionine, tryptophan, phenyl- alanine, tyrosine, and alanine ranging from 3.9-5.6 mM/5 min. The intracellular accumulation of the hydroxy-containing neutral amino acids threonine (essential) and serine (nonessential) were 2.7 mM/5 min, a value significantly lower than those of the other neutral amino acids. The accumulation of histidine was also well below the level for the other neutral amino acids (1.9 mM/5 min). The basic amino acid transport pathway was also operational with accumulation values for lysine, arginine and ornithine ranging from 1.7-2.0 mM/5 min. Accumulation of the essential amino acid lysine was not statistically different from that of nonessential ornithine. Ac- cumulation of aspartic and glutamic acid was only 0.24-0.28 mM/5 min indicating a very low activity of the acidic amino acid transport pathway. -

Parenteral Nutrition: Amino Acids
nutrients Review ParenteralReview Nutrition: Amino Acids Parenteral Nutrition: Amino Acids Leonard John Hoffer FacultyLeonard of Medicine, John Hoffer McGill University, Montreal, QC H3G 1Y5, Canada; [email protected]; Tel.:Faculty +1-514-340-8222 of Medicine, McGill University, Montreal, QC H3G 1Y5, Canada; [email protected]; Tel.: +1‐514‐340‐8222 Received: 8 February 2017; Accepted: 2 March 2017; Published: 10 March 2017 Received: 8 February 2017; Accepted: 2 March 2017; Published: date Abstract: There is growing interest in nutrition therapies that deliver a generous amount of protein, butAbstract: not a toxic There amount is growing of energy, interest to in protein-catabolic nutrition therapies critically that deliver ill patients. a generous Parenteral amount of amino protein, acids canbut achieve not a toxic this amount goal. This of energy, article to summarizes protein‐catabolic the biochemicalcritically ill patients. and nutritional Parenteral principles amino acids that guidecan parenteral achieve this amino goal. This acid article therapy, summarizes explains howthe biochemical parenteral and amino nutritional acid solutions principles are that formulated, guide andparenteral compares amino the advantages acid therapy, and explains disadvantages how parenteral of different amino parenteral acid solutions amino are acid formulated, products and with enterally-deliveredcompares the advantages whole protein and disadvantages products in the of context different of parenteral protein-catabolic amino criticalacid products illness. with enterally‐delivered whole protein products in the context of protein‐catabolic critical illness. Keywords: critical illness; parenteral nutrition; nutritional support; amino acids; protein nutrition Keywords: critical illness; parenteral nutrition; nutritional support; amino acids; protein nutrition 1. Nutritional Biochemistry of Amino Acids and Proteins 1. -

Absolute Configuration Determination
Chirality in Pharmaceutical Compounds and Absolute Configuration Determination Crystallization: Screening, Application & Process Development X-ray Diffraction Unit Jordi Benet-Buchholz Barcelona, 11 April 2019 Institut Català d’Investigació Química Av. Països Catalans 16 1 43007 Tarragona (Spain) From chiral crystals to the absolute configuration of molecules: Is it an easy way? (–)-Galiellalactone *S OH R * *S O O *R Orthorhombic , P 2 2 2 Absolute configuration: 1 1 1 R1: 3,45 % 4S, 5aR, 7aR, 7bS Flack: 0.01(19) Hooft/Parsons: -0.06(04)/-0.04(05) • F. Nussbaum, R. Hanke, T. Fahrig, J. Benet-Buchholz; Eur. J. Org. Chem. 2004, 2783-2790. 2 Determination of Absolute Configuration of APIs Definitions in Chirality Precedents Methodologies Single Crystal X-ray Structure Determination: Background Candidate samples Sample and Crystal selection Validation and measurements Examples Final schedule 3 Chirality Definitions in Chirality: An object or a system is chiral if it is distinguishable from its mirror image; that is, it cannot be superposed onto it. The word chirality is derived from the Greek word meaning “hand” Paula Benet The most universally recognized example for chirality are the human hands. Live is chiral 4 Chirality Definitions in Chirality: - Each molecule which cannot be superposed with its mirror image is “chiral”. - Molecules with a mirror plane are called “achiral”. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom (four different substituents) Two mirror images of a chiral molecule are called enantiomers or optical isomers Foto cristall papallona 5 Conglomerate of crystals forming enantiomeric butterflies Chirality Definitions in Chirality: Pairs of enantiomers are often designated as “right- or left-handed”. -

Absolute Configuration Some Definitions
Absolute Structure Absolute Configuration Some definitions • Absolute Configuration -> spatial arrangement of the atoms for a chiral molecule (R/S, P/M or D/L assignment). • Absolute Structure -> spatial arrangement of atoms in a noncentrosymmetric crystal structure (unit-cell, space group) • Chiral molecules Molecules that cannot be superimposed with their mirror image • Two mirror images of a chiral molecule are called enantiomers, they are optical isomers • Determination of absolute configuration -> handness of the molecule Origin of chirality • Asymmetric carbon atoms (R/S) • Axial chirality R-Binol S-Binol • Chiral Propeller Arrangement (P/M or / ) Helicenes In the solid state • Chiral molecules can crystallize as an enantiopure bulk sample or as a racemic mixture. • For enantiopure crystals Space group restriction: Only 65 space groups allowed for chiral molecules: No Inversion Center / No Mirror / No Glide Plane These include 11 pairs of eniantomorph space groups (screw axes of opposite handedness) eg: P41/P43 or P61/P65 Space Group Restrictions 85 % Racemic mixture In the solid state • 1) Conglomerate: a mixture of well-resolved crystals of both enantiomers Chiral space group Individual crystals have an optical activity 5-10 % • 2) Racemates: One type of crystal containing the two enantiommers in a well defined stoechiometry. No optical activity Usually centrosymmetric space group 90-95 % Conglomerate Racemates Racemic mixture In the solid state • 3) Inversion twin : twinned crystals of both enantiomers Chiral space group • 4) -

1 Fundamentals of the Stereochemistry of Organophosphorus Compounds
1 1 Fundamentals of the Stereochemistry of Organophosphorus Compounds 1.1 Historical Background Natural processes are subordinate to geometrodynamics – the theory describing physical objects, geometrical spacetime, and associated phenomena completely in terms of geometry, and her elder sister – symmetry. Symmetry/asymmetry is one of the basic concepts in modern natural science [1]. Research into this field began in the Middle Ages, when the birefringent properties of calcite were discovered. In 1669, Bartholinus observed the double refractive properties of the calcite Iceland spar. Later, in 1801, the mineralogist Haui found that quartz crystals are enantiomorphic, representing mirror images of one another. In 1815, another French naturalist J.-B. Biot discovered that certain chemical compounds rotate the plane of a beam of polarized light [2]. Biot constructed the first polarimeter and he also discovered that many natural compounds exhibit optical activity, that is, they rotate the plane of circularly polarized light. Studying crystals under a microscope, Biot discovered two types of crystals. The sample consisting of crystals of one type turned polarized light clockwise and that from another type in the opposite direction. A mixture of the two types of crystals had a neutral effect on polar- ized light. The nature of this property remained a mystery until 1848, when Louis Pasteur proposed that it had a molecular basis originating from some form of dissymmetry [3]. Pasteur separated the left and right hemihedral crystals of the sodium-ammonium salt of D,L-tartaric acid under a microscope, and connected the opposite optical activity to the mirror image of these crystals. Pasteur termed the mixture creating polarization as dissymetric and the phenomenon as dissymmetry (asymmetry). -

Distinction of Enantiomers by NMR
REVIEWS Disinction of enantiomers by NMR spectroscopy using chiral orienting media Burkhard Luy Abstract | NMR spectroscopy is a very important analytical tool in modern organic and inorganic chemistry. Next to the identification of molecules and their structure determination, it is also used for the distinction of enantiomers and the measurement of enantiomeric purity. This article gives a brief review of the techniques being developed for enantiomeric differentiation by virtue of chiral alignment media and their induction of enantiomerically dependent anisotropic NMR parameters like residual dipolar couplings. An overview of existing chiral alignment media, a brief introduction into the basic theory and measurement of the various anisotropic parameters, and several example applications are given. 1. Introduction valuable compounds one doesn’t necessarily want to NMR spectroscopy is one of the most important irreversibly modify the substance. analytical tools in modern organic and inorganic Another possibility for the distinction of chemistry as it is the only tool that allows the enantiomers is the orientation of the molecule of determination of molecular structures at atomic interest in a so-called chiral alignment medium. resolution in solution. It is used to identify the In this case, the molecule is partially aligned constitution, conformation and configuration and anisotropic NMR parameters like residual of countless molecules every day. However, the quadrupolar couplings, residual dipolar couplings magnetic field used for the induction of the Zeeman and residual chemical shift anisotropy can be splitting is per se achiral so that enantiomers measured2–4. As the orientation in a chiral have identical properties and therefore identical alignment medium is different for the two NMR spectra. -

Role and Characteristics of Selected Amino Acid and Peptide Transporters in Epithelial Cells
TECHNISCHE UNIVERSITÄT MÜNCHEN Lehrstuhl für Ernährungsphysiologie Role and characteristics of selected amino acid and peptide transporters in epithelial cells Alexander Georg Nickel Vollständiger Abdruck der von der Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt der Technischen Universität München zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigten Dissertation. Vorsitzender: Univ.-Prof. Dr. M. Schemann Prüfer der Dissertation: 1. Univ.-Prof. Dr. H. Daniel 2. Univ.-Prof. Dr. Th. F. Hofmann Die Dissertation wurde am 12.05.2009 bei der Technischen Universität München eingereicht und durch die Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt am 01.09.2009 angenommen. A 2 Zum Erfolg braucht der Forscher die vier großen "G": Geist, Geduld, Geld und Glück. Paul Ehrlich 3 Table of contents 1 Characteristics of L-proline transport in OK cells................................................................ 10 1.1 Introduction ............................................................................................................. 10 1.1.1 Physiological importance of amino acids..................................................... 10 1.1.2 Basic principles of amino acid transport...................................................... 10 1.1.3 Amino acid transport in kidney .................................................................... 11 1.1.3.1 Apical amino acid transporters of the kidney proximal tubule ..................... 12 1.1.3.2 -
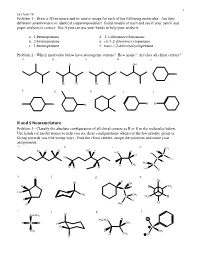
R and S Nomemclature Problem 3 - Classify the Absolute Configuration of All Chiral Centers As R Or S in the Molecules Below
1 Lecture 16 Problem 1 - Draw a 3D structure and its mirror image for each of the following molecules. Are they different (enantiomers) or identical (superimposable)? Build models of each and see if your pencil and paper analysis is correct. See if you can use your hands to help your analysis. a. 1-bromopentane d. 1,1-dibromocyclopentane b. 2-bromopentane e. cis-1,2-dibromocyclopentane c. 3-bromopentane f. trans-1,2-dibromocyclopentane Problem 2 - Which molecules below have stereogenic centers? How many? Are they all chiral centers? a. b. c. d. e. Cl OH Br Br Cl Br Br f. g. h. i. j. Br Br Br Br R and S Nomemclature Problem 3 - Classify the absolute configuration of all chiral centers as R or S in the molecules below. Use hands (or model atoms) to help you see these configurations whenever the low priority group is facing towards you (the wrong way). Find the chiral centers, assign the priorities and make your assignments. a. b. c. d. CH3 Cl H H H3C H H H H3C Br Cl C HO C CH3 I H H3C H CH3 C H H H H3C H e. O f. g. h. H Br Br OH H H H CH3 C H3C CH3 CH3 C H3C H H H H C H Cl 3 i. j. k. l. Cl CH2CH3 Br H3CH2C H C Br H CH3 Cl S CH3 D CH3 H CH3 O H 2 Lecture 16 Pi Bond Priority Problem 4 - Evaluate the order of priority in each part. -

L-Proline Cell Culture Tested, Meets EP & USP Testing Specifications
L-Proline Cell culture tested, meets EP & USP testing specifications Product Number P 5607 Product Description A procedure for the asymmetric self-aldolization of Molecular Formula: C5H9NO2 acetaldehyde that uses L-proline as a catalyst has Molecular Weight: 115.1 been reported.11 Ground- and transition-state CAS Number: 147-85-3 analogue inhibitors of cyclophilin have been 1 pKa: 1.95 (COOH), 10.64 (NH2) synthesized using L-proline as the starting pI: 6.31 compound.12 The use of L-proline to synthesize an Synonyms: (S)-Pyrrolidine-2-carboxylic acid, Pro optically active 1-oxo-2-oxa-5-azaspiro[3.4]octane compound, related to the antibiotic oxazolomycin, has This product is cell culture tested (0.03 mg/ml) and is been described.13 tested for endotoxin levels. Precautions and Disclaimer The amino acid L-Proline is unique among the For Laboratory Use Only. Not for drug, household or 20 amino acids normally found in proteins because it other uses. is the only imino acid among them.2 The side chain is bonded to both the a-carbon and nitrogen atoms, Preparation Instructions giving a secondary amino group in the structure, and This product is soluble in water (50 mg/ml), yielding a thus its characterization as an imino acid. In vivo, clear, colorless solution. proline is both synthesized from and degraded to glutamate, with glutamate g-semialdehyde and References D1-pyrroline-5-carboxylate as intermediates, by 1. Molecular Biology LabFax, Brown, T. A., ed., BIOS separate pathways.3,4 Scientific Publishers Ltd. (Oxford, UK: 1991), p. 29.