Colliding Epidemics and the Rise of Cryptococcosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mucormycosis of the Central Nervous System
Journal of Fungi Review Mucormycosis of the Central Nervous System 1 1,2, , 3, , Amanda Chikley , Ronen Ben-Ami * y and Dimitrios P Kontoyiannis * y 1 Infectious Diseases Unit, Tel Aviv Sourasky Medical Center, Tel Aviv 64239, Israel 2 Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv 64239, Israel 3 Department of Infectious Diseases, The University of Texas, M.D. Anderson Cancer Center, Houston, TX 77030, USA * Correspondence: [email protected] (R.B.-A.); [email protected] (D.P.K.) These authors contribute equally to this paper. y Received: 6 June 2019; Accepted: 7 July 2019; Published: 8 July 2019 Abstract: Mucormycosis involves the central nervous system by direct extension from infected paranasal sinuses or hematogenous dissemination from the lungs. Incidence rates of this rare disease seem to be rising, with a shift from the rhino-orbital-cerebral syndrome typical of patients with diabetes mellitus and ketoacidosis, to disseminated disease in patients with hematological malignancies. We present our current understanding of the pathobiology, clinical features, and diagnostic and treatment strategies of cerebral mucormycosis. Despite advances in imaging and the availability of novel drugs, cerebral mucormycosis continues to be associated with high rates of death and disability. Emerging molecular diagnostics, advances in experimental systems and the establishment of large patient registries are key components of ongoing efforts to provide a timely diagnosis and effective treatment to patients with cerebral mucormycosis. Keywords: central nervous system; mucormycosis; Mucorales; zygomycosis 1. Introduction Mucormycosis is the second most frequent invasive mold disease after aspergillosis [1–3], with rising incidence reported in some countries [4–7]. -

Fungal Infections – an Overview
REVIEW Fungal infections – An overview Natalie Schellack, BCur, BPharm, PhD(Pharmacy); Jade du Toit, BPharm; Tumelo Mokoena, BPharm; Elmien Bronkhorst, BPharm, MSc(Med) School of Pharmacy, Faculty of Health Sciences, Sefako Makgatho Health Sciences University Correspondence to: Prof Natalie Schellack, [email protected] Abstract Fungi normally originate from the environment that surrounds us, and appear to be harmless until inhaled or ingestion of spores occurs. A pathogenic fungus may lead to infection. People who are at risk of acquiring fungal infection are those living with human immunodeficiency virus (HIV), cancer, receiving immunosuppressant therapy, neonates and those of advanced age. The management of superficial fungal infections is mainly topical, with agents including terbinafine, miconazole and ketoconazole. Oral treatment includes griseofulvin and fluconazole. Invasive fungal infections are difficult to treat, and are managed with agents including the azoles, echinocandins and amphotericin B. This paper provides a general overview of the management of fungus infections. © Medpharm S Afr Pharm J 2019;86(1):33-40 Introduction more advanced biochemical or molecular testing.4 Fungi normally originate from the environment that surrounds Superficial fungal infections us, and appear to be harmless until inhaled or ingestion of spores Either yeasts or fungi can cause dermatomycosis, or superficial occurs. Infection with fungi is also more likely when the body’s fungal infections.7 Fungi that infect the hair, skin, nails and mucosa immune system becomes weakened. A pathogenic fungus may lead to infection. The number of fungus species ranges in the can cause a superficial fungal infection. Dermatophytes are found millions and only a few species seem to be harmful to humans; the naturally in soil, human skin and keratin-containing structures, 3 ones found mostly on the mucous membrane and the skin have which provide them with a source of nutrition. -

Cryptococcosis in Cats
12 Cryptococcosis in cats FACT SHEET What is cryptococcosis? ! Atypical forms are characterized by one or more skin nodules that are not ! Cryptococcosis is the most common systemic fungal disease in cats worldwide. painful but may be firm or fluctuant. Solitary nodules are suggestive of direct inoculation. ! It is caused by the C. neoformans-C. gattii species complex which can also infect o Multiple nodules are suggestive of haematogenous spread from the primary humans, domestic and wild mammals and birds. o site of infection. ! C. neoformans is considered an opportunistic pathogen in human urban ! populations, whereas C. gattii is a true pathogen, more prevalent in rural areas. Haematogenous dissemination may lead to meningoencephalomyelitis, uveitis, chorioretinitis, osteomyelitis, polyarthritis, systemic lymphadenitis and multi- ! Cryptococcosis is a rare non-contagious fungal disease, acquired from a organ involvement. contaminated environment. ! CNS involvement may occur following local dissemination through the cribriform plate, causing sudden blindness, seizures and/or behavioural changes. ! Apathy and cachexia appear in chronic cases with systemic dissemination. Pathogenesis ! Cryptococcus is mainly an airborne pathogen, and basidiospores, which develop in the environment, penetrate the cat’s respiratory system and induce primary Diagnosis infection. ! Cutaneous inoculation or spread from the respiratory to the central nervous ! Cytology: samples stained with Romanowsky-type stains demonstrate pink to system (CNS) is also possible. violet, round or budding yeasts that vary in size (4-15 microns) and shape. They are typically surrounded by a clear, more or less thick halo corresponding to the ! The yeast cell survives inside phagocytic cells such as macrophages, dendritic unstained capsule. cells, and neutrophils, replicating both extracellularly and intracellularly. -

Clinical Diversity of Invasive Cryptococcosis in AIDS Patients
Zhang et al. BMC Infectious Diseases (2019) 19:1003 https://doi.org/10.1186/s12879-019-4634-7 CASE REPORT Open Access Clinical diversity of invasive cryptococcosis in AIDS patients from central China: report of two cases with review of literature Yongxi Zhang1, Brian Cooper2,Xi’en Gui1, Renslow Sherer2 and Qian Cao1* Abstract Background: Although antiretroviral therapy (ART) has greatly improved the prognosis of acquired immunodeficiency syndrome (AIDS) patients globally, opportunistic infections (OIs) are still common in Chinese AIDS patients, especially cryptococcosis. Case presentation: We described here two Chinese AIDS patients with cryptococcal infections. Case one was a fifty- year-old male. At admission, he was conscious and oriented, with papulonodular and umbilicated skin lesions, some with ulceration and central necrosis resembling molluscum contagiosum. The overall impression reminded us of talaromycosis: we therefore initiated empirical treatment with amphotericin B, even though the case history of this patient did not support such a diagnosis. On the second day of infusion, the patient complained of intermittent headache, but the brain CT revealed no abnormalities. On the third day, a lumbar puncture was performed. The cerebral spinal fluid (CSF) was turbid, with slightly increased pressure. India ink staining was positive, but the cryptococcus antigen latex agglutination test (CrAgLAT: IMMY, USA) was negative. Two days later, the blood culture showed a growth of Cryptococcus neoformans, and the same result came from the skin culture. We added fluconazole to the patient’s treatment, but unfortunately, he died three days later. Case two was a sixty-four-year-old female patient with mild fever, productive cough, dyspnea upon movement, and swelling in both lower limbs. -

Valley Fever a K a Coccidioidomycosis Coccidioidosis Coccidiodal Granuloma San Joaquin Valley Fever Desert Rheumatism Valley Bumps Cocci Cox C
2019 Lung Infection Symposium - Libke 10/26/2019 58 YO ♂ • 1974 PRESENTED WITH HEADACHE – DX = COCCI MENINGITIS WITH HYDROCEPHALUS – Rx = IV AMPHOTERICIN X 6 WKS – VP SHUNT – INTRACISTERNAL AMPHO B X 2.5 YRS (>200 PUNCTURES) • 1978 – 2011 VP SHUNT REVISIONS X 5 • 1974 – 2019 GAINFULLY EMPLOYED, RAISED FAMILY, RETIRED AND CALLS OCCASIONALLY TO SEE HOW I’M DOING. VALLEY FEVER A K A COCCIDIOIDOMYCOSIS COCCIDIOIDOSIS COCCIDIODAL GRANULOMA SAN JOAQUIN VALLEY FEVER DESERT RHEUMATISM VALLEY BUMPS COCCI COX C 1 2019 Lung Infection Symposium - Libke 10/26/2019 COCCIDIOIDOMYCOSIS • DISEASE FIRST DESCRIBED IN 1892 – POSADAS –ARGENTINA – RIXFORD & GILCHRIST - CALIFORNIA – INITIALLY THOUGHT PARASITE – RESEMBLED COCCIDIA “COCCIDIOIDES” – “IMMITIS” = NOT MINOR COCCIDIOIDOMYCOSIS • 1900 ORGANISM IDENTIFIED AS FUNGUS – OPHULS AND MOFFITT – ORGANISM CULTURED FROM TISSUES OF PATIENT – LIFE CYCLE DEFINED – FULFULLED KOCH’S POSTULATES 2 2019 Lung Infection Symposium - Libke 10/26/2019 COCCIDIOIDOMYCOSIS • 1932 ORGANISM IN SOIL SAMPLE FROM DELANO – UNDER BUNKHOUSE OF 4 PATIENTS – DISEASE FATAL • 1937 DICKSON & GIFFORD CONNECTED “VALLEY FEVER” TO C. IMMITIS – USUALLY SELF LIMITED – FREQUENTLY SEEN IN SAN JOAQUIN VALLEY – RESPIRATORY TRACT THE PORTAL OF ENTRY The usual cause for coccidioidomycosis in Arizona is C. immitis A. True B. False 3 2019 Lung Infection Symposium - Libke 10/26/2019 COCCIDIOIDAL SPECIES • COCCIDIOIDES IMMITIS – CALIFORNIA • COCCIDIOIDES POSADASII – NON-CALIFORNIA • ARIZONA, MEXICO • OVERLAP IN SAN DIEGO AREA THE MICROBIAL WORLD • PRIONS -

Prostatic Cryptococcosis - a Case Report
Received: November 8, 2007 J. Venom. Anim. Toxins incl. Trop. Dis. Accepted: April 1, 2008 V.14, n.2, p.378-385, 2008. Abstract published online: April 2, 2008 Case report. Full paper published online: May 31, 2008 ISSN 1678-9199. PROSTATIC CRYPTOCOCCOSIS - A CASE REPORT CHANG M. R. (1), PANIAGO A. M. M. (2), SILVA M. M. (3), LAZÉRA M. S. (4), WANKE B. (4) (1) Department of Pharmacy-Biochemistry, Federal University of Mato Grosso do Sul (UFMS), Campo Grande, Mato Grosso do Sul State, Brazil; (2) Department of Internal Medicine, UFMS, Campo Grande, Mato Grosso do Sul State, Brazil; (3) Medicine Program, University for Development of the State and the Pantanal Region (UNIDERP), Campo Grande, Mato Grosso do Sul State, Brazil; (4) Mycology Service Evandro Chagas Institute of Clinical Research (IPEC), Oswaldo Cruz Foundation (FIOCRUZ), Rio de Janeiro, Rio de Janeiro State, Brazil. ABSTRACT: Cryptococcosis is a systemic mycosis usually affecting immunodeficient individuals. In contrast, immunologically competent patients are rarely affected. Dissemination of cryptococcosis usually involves the central nervous system, manifesting as meningitis or meningoencephalitis. Prostatic lesions are not commonly found. A case of prostate cryptococcal infection is presented and cases of prostatic cryptococcosis in normal and immunocompromised hosts are reviewed. A fifty-year-old HIV-negative man with urinary retention and renal insufficiency underwent prostatectomy due to massive enlargement of the organ. Prostate histopathologic examination revealed encapsulated yeast-like structures. After 30 days, the patient’s clinical manifestations worsened, with headache, neck stiffness, bradypsychia, vomiting and fever. Direct microscopy of the patient’s urine with China ink preparations showed capsulated yeasts, and positive culture yielded Cryptococcus neoformans. -

Pulmonary Cryptococcosis Secondary To
& My gy co lo lo ro g i y V Ramana et al., Virol Mycol 2012, 1:3 Virology & Mycology DOI: 10.4172/2161-0517.1000107 ISSN: 2161-0517 Case Report Open Access Pulmonary Cryptococcosis Secondary to Bronchial Asthma Presenting as Type I Respiratory Failure- A Case Report with Review of Literature KV Ramana*, Moses Vinay Kumar, Sanjeev D Rao, Akhila R, Sandhya, Shruthi P, Pranuthi M, Krishnappa M, Anand K Department of Microbiology, Prathima Inst of Medical Sciences, Nagunur, Karimnagar, Andhrapradesh, India Introduction mellitus, pleuritis, systemic lupus erythematus, cushings syndrome, Continous Ambulatory Peritoneal Dialysis (CAPD), liver cirrhosis, Cryptococcus spp, was first isolated and described in 1894 by cancer, organ transplants, spleenectomy, malnutrition, leprosy, Sanfelice F in Italy from peach juice and named it as Saccharomyces pulmonary tuberculosis and those on cortico steroid therapy [13-20]. neoformans, is an yeast like fungus [1], first isolated from a clinical Pulmonary Cryptococcosis may be presenting as pleural effusions, specimen by Busse in the same year from Germany [2]. Cryptococci solitary or multiple masses, glass-ground interstitial opacities, dense are a saprophytic fungi present in soil contaminated with bird consolidations, patchy, segmented or lobar air space consolidation droppings mainly of pigeons, roosting sites and decaying vegetables (cryptococcal pneumonia) and nodular and reticulonodular cavities. [3]. Previous reports have also showed the presence of Cryptococcus Differential diagnosis of pulmonary cryptococcosis should be done spp colonized in the nasopharynx and on skin of healthy individuals with pneumonia [21,22]. Review of literature showed only two previous [4]. Belonging to Basidiomyctes group of fungi Cryptococcus spp reported cases of pulmonary cryptococcosis presenting as acute primarily infects central nervous system causing meningoencephalitis respiratory failure [7,23,24]. -

Med Chem 401: Mycology Mycology
Med Chem 401: Mycology (www.doctorfungus.org) Mycology is the Study of Fungi (Monera, Protoctista, Fungi, Plantae, Animalia). Fungi are eukaryotic cells and as such contain nuclei, mitochondria, ER, golgi, 80S ribosomes, etc., bound by a plasma membrane. Note that fungal cell membranes contain ergosterol rather than cholesterol. Mycology In addition, fungi possess a rigid cell wall containing chitin, glucans and other sugar polymers. Mycology Fungi are classified as •Yeasts - round/oval cells that divide by budding •Moulds - tubular structures (hyphae) that grow by longitudinal extension and branching. A mass of hyphae is called a mycelium Diseases Caused by Fungi Fungal infections in normal healthy adults are confined to conditions such as mucosal candidiasis (e.g., thrush) and dermatophyte (tinea) skin infections (e.g., athlete's foot). However, in the immunocompromised host, a variety of normally mild or nonpathogenic fungi can cause potentially fatal infections. Diseases Caused by Fungi Fungal infections are classified depending on the degree of tissue involvement and mode of entry: 1. Superficial - localized to the skin, hair and nails. 2. Subcutaneous - infection confined to the dermis, subcutaneous tissue, or adjacent structures. 3. Systemic - deep infections of the internal organs. 4. Opportunistic - cause infection only in the immunocompromised. 1. Superficial Mycoses The Dermatophytes The dermatophytes are not a specific fungus, but rather a short-hand label for three genera of fungi that commonly cause skin disease (tinea). •Epidermophyton spp. •tinea capitis •tinea barbae •Trichophyton spp. •tinea pedis •Microsporum spp. •tinea cruis 1. Superficial Mycoses The Dermatophytes Tinea pedis “athletes foot” Tinea capitis Epidermophyton spp. Microsporum spp. 1. -
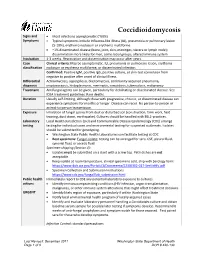
Coccidioidomycosis Reporting and Investigation Guideline
Coccidioidomycosis Signs and • Most infections asymptomatic (~60%) Symptoms • Typical symptoms include influenza-like illness (ILI), pneumonia or pulmonary lesion (5-10%), erythema nodosum or erythema multiforme • ~1% disseminated disease (bone, joint, skin, meninges, viscera or lymph node); dissemination more likely for men, some racial groups, altered immune system Incubation 1-3 weeks. Reactivation and dissemination may occur after years. Case Clinical criteria: May be asymptomatic. ILI, pneumonia or pulmonary lesion, erythema classification nodosum or erythema multiforme, or disseminated infection. Confirmed: Positive IgM, positive IgG, positive culture, or skin-test conversion from negative to positive after onset of clinical illness Differential Actinomycosis, aspergillosis, blastomycosis, community acquired pneumonia, diagnosis cryptococcosis, histoplasmosis, meningitis, sarcoidosis, tuberculosis, malignancy Treatment Antifungal agents can be given, particularly for debilitating or disseminated disease. See IDSA treatment guidelines. Rare deaths. Duration Usually self-limiting, although those with progressive, chronic, or disseminated disease can experience symptoms for months or longer. Disease can recur. No person-to-person or animal-to-person transmission. Exposure Inhalation of fungal spores from dust or disturbed soil (construction, farm work, field training, dust storm, earthquake). Cultures should be handled with BSL2-practices. Laboratory Local Health Jurisdiction (LHJ) and Communicable Disease Epidemiology (CDE) arrange testing testing for individual cases and environmental testing for suspected outbreaks. Isolates should be submitted for genotyping. • Washington State Public Health Laboratories can facilitate testing at CDC • Best specimens: Fungal isolate; testing can be arranged for sera, CSF, pleural fluid, synovial fluid, or ascetic fluid Specimen shipping (Section 4): • Isolates must be submitted on a slant with a screw top. Petri dishes are not acceptable. -

Black Fungus: a New Threat Uddin KN
Editorial (BIRDEM Med J 2021; 11(3): 164-165) Black fungus: a new threat Uddin KN Fungal infections, also known as mycoses, are Candida spp. including non-albicans Candida (causing traditionally divided into superficial, subcutaneous and candidiasis), p. Aspergillus spp. (causing aspergillosis), systemic mycoses. Cryptococcus (causing cryptococcosis), Mucormycosis previously called zygomycosis caused by Zygomycetes. What are systemic mycoses? These fungi are found in or on normal skin, decaying Systemic mycoses are fungal infections affecting vegetable matter and bird droppings respectively but internal organs. In the right circumstances, the fungi not exclusively. They are present throughout the world. enter the body via the lungs, through the gut, paranasal sinuses or skin. The fungi can then spread via the Who are at risk of systemic mycoses? bloodstream to multiple organs, often causing multiple Immunocompromised people are at risk of systemic organs to fail and eventually, result in the death of the mycoses. Immunodeficiency can result from: human patient. immunodeficiency virus (HIV) infection, systemic malignancy (cancer), neutropenia, organ transplant What causes systemic mycoses? recipients including haematological stem cell transplant, Patients who are immunocompromised are predisposed after a major surgical operation, poorly controlled to systemic mycoses but systemic mycosis can develop diabetes mellitus, adult-onset immunodeficiency in otherwise healthy patients. Systemic mycoses can syndrome, very old or very young. be split between two main varieties, endemic respiratory infections and opportunistic infections. What are the clinical features of systemic mycoses? The clinical features of a systemic mycosis depend on Endemic respiratory infections the specific infection and which organs have been Fungi that can cause systemic infection in people with affected. -

Clinical Aspects of Blastomycosis
Thorax: first published as 10.1136/thx.25.6.708 on 1 November 1970. Downloaded from Thorax (1970), 25, 708. Clinical aspects of blastomycosis RICHARD P. O'NEILL and ROBERT W. B. PENMAN Department of Medicine, University of Kentucky Medical Center, Lexington, Kentucky Blastomycosis is a specific granulomatous disease which tends to be chronic and indolent. It frequently presents in extrapulmonary form by means of haematogenous dissemination from the lungs. It has been shown that tuberculosis, histoplasmosis and coccidioidomycosis are, in the majority of cases, mild and subclinical in effect and often heal without therapy. It is probable that blastomycosis behaves in a like manner. The exact mortality is not known but is probably in the range of 13% in hospitalized cases with disseminated disease (Blastomycosis Cooperative Study of the Veterans Administration, 1964). The most effective form of therapy in active disease is amphotericin B; 2-hydroxy-stilbamidine is also used. Blastomycosis has largely been considered to be a disease of the American continent. However, cases have been reported from Africa and Europe and therefore a wider appreciation of this disease is considered pertinent. The relevant literature has been reviewed and four illustrative cases are presented. North American blastomycosis was first described 100,000 population (Furcolow et al., 1966). How- by Gilchrist in 1896 in a report of a case which ever, recent studies have shown previously un- had previously been diagnosed as 'pseudolupus recognized, widely separated areas of endemic copyright. vulgaris' and had been thought to be tuberculous blastomycosis. Seven cases have been reported in origin. Gilchrist showed that the disease was from Africa; two from the Congo (Gatti, caused by a specific organism, Blastomyces derma- Renoirte, and Vandepitte, 1964; Gatti, De Broe, titidis. -

Primary Capsule-Deficient Cutaneous Cryptococcosis in a Sporotrichoid Pattern in an Immunocompetent Host
Primary Capsule-Deficient Cutaneous Cryptococcosis in a Sporotrichoid Pattern in an Immunocompetent Host Nathan Andrew Merl Jackson, DO; Daniel B. Herring, MD Practice Points Cryptococcus neoformans is an encapsulated yeast that is ubiquitous in the environment and is especially abundant in soil enriched with pigeon droppings. Immunocompetent hosts often are asymptomatic or have only mild pulmonary disease, while disseminated disease affects the lungs, central nervous system, bones, and skin in immunocompromised hosts. Diagnostic tests include india ink or mucicarmine staining to highlight characteristic capsules or the latex agglutination test to measure circulating capsular antigen. copy not Cryptococcosis is an opportunistic yeast infectionDo ryptococcosis is an opportunistic yeast infection caused by Cryptococcus neoformans that remains caused by Cryptococcus neoformans that remains the most common systemic fungal infection in Cthe most common systemic fungal infection in immunosuppressed patients and often pres- immunosuppressed patients and often presents with ents with signs of meningitis. Primary cutaneous signs of meningitis. Cutaneous cryptococcosis occurs cryptococcosis (PCC) is a more rare clinical iden- in 10% to 20% of systemic Cryptococcus infections and tity that is characterized by skin lesions confined usually is secondary to hematogenous dissemination in to 1 body region, often presenting as a whitlow or patients with an underlying disease, particularly human phlegmon with positive cultureCUTIS for C neoformans immunodeficiency virus. Primary cutaneous crypto- and no evidence of simultaneous dissemination. coccosis (PCC) is a more rare clinical identity that We report a rare case of PCC in a 73-year-old man is characterized by skin lesions confined to 1 body with intact cell-mediated immunity.